HLA-B*57:01 allele prevalence in treatment-Naïve HIV-infected patients from Colombia
- PMID: 31500584
- PMCID: PMC6734234
- DOI: 10.1186/s12879-019-4415-3
HLA-B*57:01 allele prevalence in treatment-Naïve HIV-infected patients from Colombia
Abstract
Background: The HLA-B*57:01 allele is associated with a hypersensitivity reaction to abacavir. Due to the lack of knowledge of HLA-B*57:01 prevalence in Colombia, routine screening is not performed and is not recommended by the national guidelines. We aimed to determine the prevalence of HLA-B*57:01 in HIV population from Colombia.
Methods: This cross-sectional study included naïve HIV-infected adults from 13 cities of the country. The presence of HLA-B*57:01 was determined by using SSP-PCR in blood samples. Prevalence rates were stratified by sex, race, and region of origin.
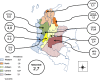
Results: HLA-B*57:01 allele prevalence in Colombian HIV-infected individuals was 2.7%. When stratifying for the race, the prevalence was 4% for whites, 2.6% for other race (mainly mestizo), and 1.9% for Afro-Colombians. The prevalence varied from 0% up to 11.4% depending on the department of origin. The highest prevalence rates were found in Caldas (11.4%), Antioquia (5%), Risaralda (4.8%), and Valle del Cauca (4.3%). When distributed by country zones, the central, with a racial predominance of Caucasians and mestizos, was the highest (6.0%, 0R = 4.1, CI 1.2-12.8, p = 0,016).
Conclusions: The overall prevalence of HLA-B*57:01 in Colombia was lower than the reported rates for other Latin American countries such as Brazil, Costa Rica, and Argentina, but similar in comparison to Chile and Mexico. The diversity in the racial and ethnic heritage shown in our data supports the recommendation to implement routine screening for the HLA-B*57:01 allele before initiation of abacavir-containing antiretroviral therapy in the Colombian HIV management guidelines.
Keywords: Abacavir; Antiretroviral therapy; HIV infection; HLA-B*57:01; Hypersensitivity; Pharmacogenetics. Cross-sectional study.
Conflict of interest statement
EMB reports research grants from ViiV Healthcare/GSK, financial support for REVIVA foundation and VIHCOL Group (Grupo Colombiano de VIH) from GSK, MSD and Stendhal, and personal fees for presentations and advisory board meetings from GSK, MSD and Stendhal and presentations from Janssen Pharmaceuticals. JMO reports grants from ViiV Healthcare/GlaxoSmithKline, and personal fees from Merck Sharp & Dohme Colombia. JFO reports personal fees from Merck Sharp and Dohme Colombia. JA reports grants from ViiV Healthcare/GlaxoSmithKline and grants and personal fees from Merck Sharp & Dohme Colombia SAS. WL reports grants from ViiV Healthcare/GlaxoSmithKline, personal fees from Janssen Cilag SA, and Merck Sharp & Dohme Colombia; LR reports grants from ViiV Healthcare/GSK and Merck Sharp & Dohme Colombia. LMS declares no conflicts of interest.
Figures
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. (Updated 25 October 2018). https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. (2018).
-
- European AIDS Clinical Society. European AIDS Clinical Society Guidelines Version 9.1., Updated October 2018. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. (2018).
-
- Ministry of Health and Social Protection. Evidence-based clinical practice guidelines for HIV infection care of teenagers (13 years old and older) and adults. (2014).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials