Heterogeneity of human pancreatic β-cells
- PMID: 31500834
- PMCID: PMC6768494
- DOI: 10.1016/j.molmet.2019.06.015
Heterogeneity of human pancreatic β-cells
Abstract
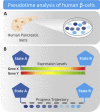
Background: Human pancreatic β-cells are heterogeneous. This has been known for a long time and is based on various functional and morphological readouts. β-Cell heterogeneity could reflect fixed subpopulations with distinct functions. However, recent pseudotime analysis of large-scale RNA sequencing data suggest that human β-cell subpopulations may rather reflect dynamic interchangeable states characterized by low expression of genes involved in the unfolded protein response (UPR) and low insulin gene expression, low UPR and high insulin expression or high UPR and low insulin expression.
Scope of review: This review discusses findings obtained by single-cell RNA sequencing combined with pseudotime analysis that human β-cell heterogeneity represents dynamic interchangeable functional states. The physiological significance and potential implications of β-cell heterogeneity in the development and progression of diabetes is highlighted.
Major conclusions: The existence of dynamic functional states allow β-cells to transition between periods of high insulin production and UPR-mediated stress recovery. The recovery state is important since proinsulin is a misfolding-prone protein, making its biosynthesis in the endoplasmic reticulum a stressful event. The transition of β-cells between dynamic states is likely controlled at multiple levels and influenced by the microenvironment within the pancreatic islets. Disturbances in the ability of the β-cells to transition between periods of high insulin biosynthesis and UPR-mediated stress recovery may contribute to diabetes development. Diabetes medications that restore the ability of the β-cells to transition between the functional states should be considered.
Keywords: Diabetes; Heterogeneity; Human β-cell; Insulin; Pancreatic islet.
Copyright © 2019. Published by Elsevier GmbH.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

