Development and validation of a patient-reported outcome tool to assess cancer-related financial toxicity in Italy: a protocol
- PMID: 31501130
- PMCID: PMC6738930
- DOI: 10.1136/bmjopen-2019-031485
Development and validation of a patient-reported outcome tool to assess cancer-related financial toxicity in Italy: a protocol
Abstract
Introduction: Financial toxicity (FT) is a well-recognised problem in oncology. US-based studies have shown that: (a) cancer patients have a 2.7 times risk of bankruptcy; (b) patients who declare bankruptcy have a 79% greater hazard of death; (c) financial burden significantly impairs quality of life (QoL) and (d) reduces compliance and adherence to treatment prescriptions. The aim of the project is to develop and validate a patient-reported-outcome (PRO) measure to assess FT of cancer patients in Italy, where, despite the universal health coverage provided by the National Health Service, FT is an emerging issue.
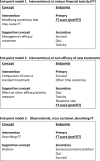
Methods and analysis: Our hypothesis is that a specific FT measure, which considers the relevant sociocultural context and healthcare system, would allow us to understand the main determinants of cancer-related FT in Italy, in order to address and reduce these factors. According to the International Society for Pharmaco-economics and Outcomes Research guidelines on PROs, the project will include the following steps: (1) concept elicitation (from focus groups with patients and caregivers; literature; oncologists; nurses) and analysis, creating a coding library; (2) item generation (using a format that includes a question and a response on a 4-point Likert scale) and analysis through patients' cognitive interviews of item importance within different coding categories to produce the draft instrument; (3) factor analysis and internal validation (with Cronbach's alpha and test-retest for reliability) to produce the final instrument; (4) external validation with QoL anchors and depression scales. The use of the FT measure in prospective trials is also planned.
Ethics and dissemination: The protocol is approved by the ethical committees of all the participating centres. The project will tentatively produce a validated tool by the spring 2021. The project might also represent a model and the basis for future cooperation with other European countries, with different healthcare systems and socioeconomic conditions.
Trial registration number: NCT03473379.
Keywords: Cancer; Financial toxicity; Health economics; Italy; Patient Reported Outcome Measures.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SR has received personal fees from CSL-Behring and GlaxoSmithKline Foundation. JB has received personal fees from Novartis, AstraZeneca, Merck Sharp & Dohme. MDM has received personal fees from Bristol Myers Squibb, Merck Sharp & Dohme, Roche, AstraZeneca, Janssen. FE has received personal fees from Bristol Myers Squibb, Incyte, Orsenyx and Amgen. LG has received personal fees from Bristol Myers Squibb. CJ has received personal fees from Amgen, Astra Zeneca, Biogen, Boehringer Ingelheim, Celgene, Gilead, GSK, Ipsen, Janssen-Cilag, Takeda and Sanofi. VM has received personal fees from Bristol Myers Squibb and Italfarmaco; a member of his family is employee in Bayer. CMV has received personal fees from Baxter, MSD, Novartis, Sanofi, Sanofi Genzyme. FP has received personal fees from Bayer, Ipsen, Astra Zeneca, Bristol Myers Squibb, Sandoz, Incyte, Celgene, Pierre Fabre, Janssen-Cilag.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous