Epithelial Lining Fluid and Plasma Concentrations of Dalbavancin in Healthy Adults after a Single 1,500-Milligram Infusion
- PMID: 31501147
- PMCID: PMC6811436
- DOI: 10.1128/AAC.01024-19
Epithelial Lining Fluid and Plasma Concentrations of Dalbavancin in Healthy Adults after a Single 1,500-Milligram Infusion
Abstract
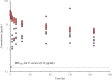
Dalbavancin is a lipoglycopeptide antibiotic with a prolonged half-life. A phase 1 study assessed dalbavancin levels in epithelial lining fluid (ELF) in 35 healthy adults using ELF bronchial microsampling up to 168 h after administration of 1,500 mg dalbavancin. The penetration of dalbavancin into ELF was 36%. ELF levels of dalbavancin exceeded the MIC90s of Streptococcus pneumoniae and Staphylococcus aureus for ≥7 days.
Keywords: dalbavancin; lung epithelial lining fluid; pharmacodynamics; pharmacokinetics; pneumonia.
Copyright © 2019 Rappo et al.
Figures
References
-
- Dunne M, Koeth LM, Windau AR. 2012. The effect of lung surfactant on the in vitro activity of dalbavancin against Staphylococcus aureus and Streptococcus pneumoniae, poster 1622. ID Week, San Diego, CA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical