Coxiella burnetii Intratracheal Aerosol Infection Model in Mice, Guinea Pigs, and Nonhuman Primates
- PMID: 31501249
- PMCID: PMC6867829
- DOI: 10.1128/IAI.00178-19
Coxiella burnetii Intratracheal Aerosol Infection Model in Mice, Guinea Pigs, and Nonhuman Primates
Abstract
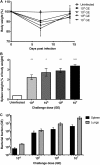
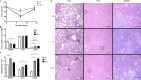
Coxiella burnetii, the etiological agent of Q fever, is a Gram-negative bacterium transmitted to humans by inhalation of contaminated aerosols. Acute Q fever is often self-limiting, presenting as a febrile illness that can result in atypical pneumonia. In some cases, Q fever becomes chronic, leading to endocarditis that can be life threatening. The formalin-inactivated whole-cell vaccine (WCV) confers long-term protection but has significant side effects when administered to presensitized individuals. Designing new vaccines against C. burnetii remains a challenge and requires the use of clinically relevant modes of transmission in appropriate animal models. We have developed a safe and reproducible C. burnetii aerosol challenge in three different animal models to evaluate the effects of pulmonary acquired infection. Using a MicroSprayer aerosolizer, BL/6 mice and Hartley guinea pigs were infected intratracheally with C. burnetii Nine Mile phase I (NMI) and demonstrated susceptibility as determined by measuring bacterial growth in the lungs and subsequent dissemination to the spleen. Histological analysis of lung tissue showed significant pathology associated with disease, which was more severe in guinea pigs. Infection using large-particle aerosol (LPA) delivery was further confirmed in nonhuman primates, which developed fever and pneumonia. We also demonstrate that vaccinating mice and guinea pigs with WCV prior to LPA challenge is capable of eliciting protective immunity that significantly reduces splenomegaly and the bacterial burden in spleen and lung tissues. These data suggest that these models can have appreciable value in using the LPA delivery system to study pulmonary Q fever pathogenesis as well as designing vaccine countermeasures to C. burnetii aerosol transmission.
Keywords: Coxiella burnetii; MicroSprayer; Q fever; aerosol; intratracheal; large-particle aerosol.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Soluble antigens derived from Coxiella burnetii elicit protective immunity in three animal models without inducing hypersensitivity.Cell Rep Med. 2021 Dec 6;2(12):100461. doi: 10.1016/j.xcrm.2021.100461. eCollection 2021 Dec 21. Cell Rep Med. 2021. PMID: 35028605 Free PMC article.
-
Coxiella burnetii Nine Mile phase I primary infection derived protective immunity against C. burnetii reinfection in mice depends on both B and T cells, but T cells play a critical role.Front Immunol. 2024 Oct 14;15:1427822. doi: 10.3389/fimmu.2024.1427822. eCollection 2024. Front Immunol. 2024. PMID: 39469719 Free PMC article.
-
Comparative efficacy of a Coxiella burnetii chloroform:methanol residue (CMR) vaccine and a licensed cellular vaccine (Q-Vax) in rodents challenged by aerosol.Vaccine. 1997 Nov;15(16):1779-83. doi: 10.1016/s0264-410x(97)00107-2. Vaccine. 1997. PMID: 9364683
-
Components of protective immunity.Adv Exp Med Biol. 2012;984:91-104. doi: 10.1007/978-94-007-4315-1_5. Adv Exp Med Biol. 2012. PMID: 22711628 Review.
-
Vaccines against coxiellosis and Q fever. Development of a chloroform:methanol residue subunit of phase I Coxiella burnetti for the immunization of animals.Ann N Y Acad Sci. 1992 Jun 16;653:88-111. doi: 10.1111/j.1749-6632.1992.tb19633.x. Ann N Y Acad Sci. 1992. PMID: 1626897 Review.
Cited by
-
Proteomic Analysis of Non-human Primate Peripheral Blood Mononuclear Cells During Burkholderia mallei Infection Reveals a Role of Ezrin in Glanders Pathogenesis.Front Microbiol. 2021 Apr 22;12:625211. doi: 10.3389/fmicb.2021.625211. eCollection 2021. Front Microbiol. 2021. PMID: 33967974 Free PMC article.
-
Subunit Vaccines Using TLR Triagonist Combination Adjuvants Provide Protection Against Coxiella burnetii While Minimizing Reactogenic Responses.Front Immunol. 2021 Mar 17;12:653092. doi: 10.3389/fimmu.2021.653092. eCollection 2021. Front Immunol. 2021. PMID: 33815413 Free PMC article.
-
Q Fever Vaccines: Unveiling the Historical Journey and Contemporary Innovations in Vaccine Development.Vaccines (Basel). 2025 Jan 31;13(2):151. doi: 10.3390/vaccines13020151. Vaccines (Basel). 2025. PMID: 40006698 Free PMC article. Review.
-
Mice fatal pneumonia model induced by less-virulent Streptococcus pneumoniae via intratracheal aerosolization.Future Microbiol. 2024 Aug 12;19(12):1055-1070. doi: 10.1080/17460913.2024.2355738. Epub 2024 Jun 24. Future Microbiol. 2024. PMID: 38913747 Free PMC article.
-
Soluble antigens derived from Coxiella burnetii elicit protective immunity in three animal models without inducing hypersensitivity.Cell Rep Med. 2021 Dec 6;2(12):100461. doi: 10.1016/j.xcrm.2021.100461. eCollection 2021 Dec 21. Cell Rep Med. 2021. PMID: 35028605 Free PMC article.
References
-
- Derrick EH. 1973. The course of infection with Coxiella burneti. Med J Aust 1:1051–1057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

