Precise Immunodetection of PTEN Protein in Human Neoplasia
- PMID: 31501265
- PMCID: PMC6886455
- DOI: 10.1101/cshperspect.a036293
Precise Immunodetection of PTEN Protein in Human Neoplasia
Abstract
PTEN is a major tumor-suppressor protein whose expression and biological activity are frequently diminished in sporadic or inherited cancers. PTEN gene deletion or loss-of-function mutations favor tumor cell growth and are commonly found in clinical practice. In addition, diminished PTEN protein expression is also frequently observed in tumor samples from cancer patients in the absence of PTEN gene alterations. This makes PTEN protein levels a potential biomarker parameter in clinical oncology, which can guide therapeutic decisions. The specific detection of PTEN protein can be achieved by using highly defined anti-PTEN monoclonal antibodies (mAbs), characterized with precision in terms of sensitivity for the detection technique, specificity for PTEN binding, and constraints of epitope recognition. This is especially relevant taking into consideration that PTEN is highly targeted by mutations and posttranslational modifications, and different PTEN protein isoforms exist. The precise characterization of anti-PTEN mAb reactivity is an important step in the validation of these reagents as diagnostic and prognostic tools in clinical oncology, including their routine use in analytical immunohistochemistry (IHC). Here, we review the current status on the use of well-defined anti-PTEN mAbs for PTEN immunodetection in the clinical context and discuss their potential usefulness and limitations for a more precise cancer diagnosis and patient benefit.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
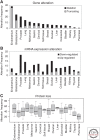

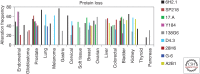

Similar articles
-
Precise definition of PTEN C-terminal epitopes and its implications in clinical oncology.NPJ Precis Oncol. 2019 Apr 15;3:11. doi: 10.1038/s41698-019-0083-4. eCollection 2019. NPJ Precis Oncol. 2019. PMID: 30993208 Free PMC article.
-
Novel anti-PTEN C2 domain monoclonal antibodies to analyse the expression and function of PTEN isoform variants.PLoS One. 2023 Aug 1;18(8):e0289369. doi: 10.1371/journal.pone.0289369. eCollection 2023. PLoS One. 2023. PMID: 37527256 Free PMC article.
-
Can we accurately report PTEN status in advanced colorectal cancer?BMC Cancer. 2014 Feb 25;14:128. doi: 10.1186/1471-2407-14-128. BMC Cancer. 2014. PMID: 24564252 Free PMC article. Clinical Trial.
-
Emerging role of PTEN loss in evasion of the immune response to tumours.Br J Cancer. 2020 Jun;122(12):1732-1743. doi: 10.1038/s41416-020-0834-6. Epub 2020 Apr 24. Br J Cancer. 2020. PMID: 32327707 Free PMC article. Review.
-
PTEN hamartoma tumour syndrome: what happens when there is no PTEN germline mutation?Hum Mol Genet. 2020 Oct 20;29(R2):R150-R157. doi: 10.1093/hmg/ddaa127. Hum Mol Genet. 2020. PMID: 32568377 Free PMC article. Review.
Cited by
-
Emerging Biomarkers in Thyroid Practice and Research.Cancers (Basel). 2021 Dec 31;14(1):204. doi: 10.3390/cancers14010204. Cancers (Basel). 2021. PMID: 35008368 Free PMC article. Review.
-
Potentiation by Protein Synthesis Inducers of Translational Readthrough of Pathogenic Premature Termination Codons in PTEN Isoforms.Cancers (Basel). 2024 Aug 13;16(16):2836. doi: 10.3390/cancers16162836. Cancers (Basel). 2024. PMID: 39199607 Free PMC article.
-
Pan-cancer genomic analysis shows hemizygous PTEN loss tumors are associated with immune evasion and poor outcome.Sci Rep. 2023 Mar 28;13(1):5049. doi: 10.1038/s41598-023-31759-6. Sci Rep. 2023. PMID: 36977733 Free PMC article.
-
Toward Systems Pathology for PTEN Diagnostics.Cold Spring Harb Perspect Med. 2020 May 1;10(5):a037127. doi: 10.1101/cshperspect.a037127. Cold Spring Harb Perspect Med. 2020. PMID: 31615872 Free PMC article. Review.
-
PTEN Function at the Interface between Cancer and Tumor Microenvironment: Implications for Response to Immunotherapy.Int J Mol Sci. 2020 Jul 27;21(15):5337. doi: 10.3390/ijms21155337. Int J Mol Sci. 2020. PMID: 32727102 Free PMC article. Review.
References
-
- Ágoston EI, Micsik T, Ács B, Fekete K, Hahn O, Baranyai Z, Dede K, Bodoky G, Bursics A, Kulka J, et al. 2016. In depth evaluation of the prognostic and predictive utility of PTEN immunohistochemistry in colorectal carcinomas: Performance of three antibodies with emphasis on intracellular and intratumoral heterogeneity. Diagn Pathol 11: 61 10.1186/s13000-016-0508-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
