Human papillomavirus E7 oncoprotein targets RNF168 to hijack the host DNA damage response
- PMID: 31501315
- PMCID: PMC6765264
- DOI: 10.1073/pnas.1906102116
Human papillomavirus E7 oncoprotein targets RNF168 to hijack the host DNA damage response
Abstract
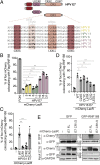
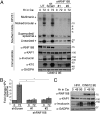
High-risk human papillomaviruses (HR-HPVs) promote cervical cancer as well as a subset of anogenital and head and neck cancers. Due to their limited coding capacity, HPVs hijack the host cell's DNA replication and repair machineries to replicate their own genomes. How this host-pathogen interaction contributes to genomic instability is unknown. Here, we report that HPV-infected cancer cells express high levels of RNF168, an E3 ubiquitin ligase that is critical for proper DNA repair following DNA double-strand breaks, and accumulate high numbers of 53BP1 nuclear bodies, a marker of genomic instability induced by replication stress. We describe a mechanism by which HPV E7 subverts the function of RNF168 at DNA double-strand breaks, providing a rationale for increased homology-directed recombination in E6/E7-expressing cervical cancer cells. By targeting a new regulatory domain of RNF168, E7 binds directly to the E3 ligase without affecting its enzymatic activity. As RNF168 knockdown impairs viral genome amplification in differentiated keratinocytes, we propose that E7 hijacks the E3 ligase to promote the viral replicative cycle. This study reveals a mechanism by which tumor viruses reshape the cellular response to DNA damage by manipulating RNF168-dependent ubiquitin signaling. Importantly, our findings reveal a pathway by which HPV may promote the genomic instability that drives oncogenesis.
Keywords: 53BP1 nuclear bodies; DNA double-strand break; E7 protein; RNF168; high-risk human papillomavirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Human papillomavirus type 16 E6 and E7 oncoproteins interact with the nuclear p53-binding protein 1 in an in vitro reconstructed 3D epithelium: new insights for the virus-induced DNA damage response.Virol J. 2018 Nov 16;15(1):176. doi: 10.1186/s12985-018-1086-4. Virol J. 2018. PMID: 30445982 Free PMC article.
-
High-Risk Alphapapillomavirus Oncogenes Impair the Homologous Recombination Pathway.J Virol. 2017 Sep 27;91(20):e01084-17. doi: 10.1128/JVI.01084-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768872 Free PMC article.
-
Tumors overexpressing RNF168 show altered DNA repair and responses to genotoxic treatments, genomic instability and resistance to proteotoxic stress.Oncogene. 2017 Apr 27;36(17):2405-2422. doi: 10.1038/onc.2016.392. Epub 2016 Nov 14. Oncogene. 2017. PMID: 27841863
-
Carcinogenesis Associated with Human Papillomavirus Infection. Mechanisms and Potential for Immunotherapy.Biochemistry (Mosc). 2019 Jul;84(7):782-799. doi: 10.1134/S0006297919070095. Biochemistry (Mosc). 2019. PMID: 31509729 Review.
-
Mechanisms of genomic instability in human cancer: insights from studies with human papillomavirus oncoproteins.Int J Cancer. 2004 Mar 20;109(2):157-62. doi: 10.1002/ijc.11691. Int J Cancer. 2004. PMID: 14750163 Review.
Cited by
-
HPV16 E6-induced M2 macrophage polarization in the cervical microenvironment via exosomal miR-204-5p.Sci Rep. 2024 Oct 10;14(1):23725. doi: 10.1038/s41598-024-74399-0. Sci Rep. 2024. PMID: 39390116 Free PMC article.
-
Nuclear antiviral innate responses at the intersection of DNA sensing and DNA repair.Trends Microbiol. 2022 Nov;30(11):1056-1071. doi: 10.1016/j.tim.2022.05.004. Epub 2022 May 28. Trends Microbiol. 2022. PMID: 35641341 Free PMC article. Review.
-
The HPV Induced Cancer Resource (THInCR): a Suite of Tools for Investigating HPV-Dependent Human Carcinogenesis.mSphere. 2022 Aug 31;7(4):e0031722. doi: 10.1128/msphere.00317-22. Epub 2022 Aug 11. mSphere. 2022. PMID: 35950764 Free PMC article.
-
Molecular aspects of cervical cancer: a pathogenesis update.Front Oncol. 2024 Mar 19;14:1356581. doi: 10.3389/fonc.2024.1356581. eCollection 2024. Front Oncol. 2024. PMID: 38567159 Free PMC article. Review.
-
Spatial and Functional Organization of Human Papillomavirus Replication Foci in the Productive Stage of Infection.mBio. 2021 Dec 21;12(6):e0268421. doi: 10.1128/mBio.02684-21. Epub 2021 Nov 9. mBio. 2021. PMID: 34749533 Free PMC article.
References
-
- de Martel C., et al. , Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 13, 607–615 (2012). - PubMed
-
- de Villiers E. M., Fauquet C., Broker T. R., Bernard H. U., zur Hausen H., Classification of papillomaviruses. Virology 324, 17–27 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

