Direct neuronal reprogramming of olfactory ensheathing cells for CNS repair
- PMID: 31501413
- PMCID: PMC6733847
- DOI: 10.1038/s41419-019-1887-4
Direct neuronal reprogramming of olfactory ensheathing cells for CNS repair
Abstract
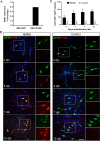
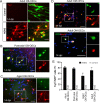
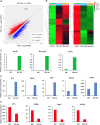
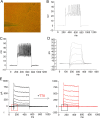
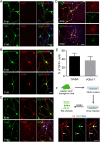
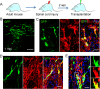
Direct conversion of readily available non-neural cells from patients into induced neurons holds great promise for neurological disease modeling and cell-based therapy. Olfactory ensheathing cells (OECs) is a unique population of glia in olfactory nervous system. Based on the regeneration-promoting properties and the relative clinical accessibility, OECs are attracting increasing attention from neuroscientists as potential therapeutic agents for use in neural repair. Here, we report that OECs can be directly, rapidly and efficiently reprogrammed into neuronal cells by the single transcription factor Neurogenin 2 (NGN2). These induced cells exhibit typical neuronal morphologies, express multiple neuron-specific markers, produce action potentials, and form functional synapses. Genome-wide RNA-sequencing analysis shows that the transcriptome profile of OECs is effectively reprogrammed towards that of neuronal lineage. Importantly, these OEC-derived induced neurons survive and mature after transplantation into adult mouse spinal cords. Taken together, our study provides a direct and efficient strategy to quickly obtain neuronal cells from adult OECs, suggestive of promising potential for personalized disease modeling and cell replacement-mediated therapeutic approaches to neurological disorders.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

