The role of pyroptosis in cancer: pro-cancer or pro-"host"?
- PMID: 31501419
- PMCID: PMC6733901
- DOI: 10.1038/s41419-019-1883-8
The role of pyroptosis in cancer: pro-cancer or pro-"host"?
Abstract
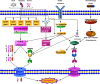
Programmed cell death (PCD) refers to the way in which cells die depending on specific genes encoding signals or activities. Apoptosis, autophagy, and pyroptosis are all mechanisms of PCD. Among these mechanisms, pyroptosis is mediated by the gasdermin family, accompanied by inflammatory and immune responses. The relationship between pyroptosis and cancer is complex, and the effects of pyroptosis on cancer vary in different tissues and genetic backgrounds. On one hand, pyroptosis can inhibit the occurrence and development of tumors; on the other hand, as a type of proinflammatory death, pyroptosis can form a suitable microenvironment for tumor cell growth and thus promote tumor growth. In addition, the induction of tumor pyroptosis is also considered a potential cancer treatment strategy. Studies have shown that DFNA5 (nonsyndromic hearing impairment protein 5)/GSDME (Gasdermin-E) mRNA methylation results in lower expression levels of DFNA5/GSDME in most tumor cells than in normal cells, making it difficult to activate the pyroptosis in most tumor cells. During the treatment of malignant tumors, appropriate chemotherapeutic drugs can be selected according to the expression levels of DFNA5/GSDME, which can be upregulated in tumor cells, thereby increasing the sensitivity to chemotherapeutic drugs and reducing drug resistance. Therefore, induced pyroptosis may play a predominant role in the treatment of cancer. Here, we review the latest research on the anti- and protumor effects of pyroptosis and its potential applications in cancer treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

