A first experience of transduction for differentiated HepaRG cells using lentiviral technology
- PMID: 31501487
- PMCID: PMC6733867
- DOI: 10.1038/s41598-019-49402-8
A first experience of transduction for differentiated HepaRG cells using lentiviral technology
Erratum in
-
Publisher Correction: A first experience of transduction for differentiated HepaRG cells using lentiviral technology.Sci Rep. 2021 Sep 6;11(1):18051. doi: 10.1038/s41598-021-97243-1. Sci Rep. 2021. PMID: 34489522 Free PMC article. No abstract available.
Abstract
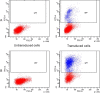
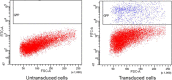
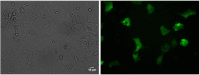
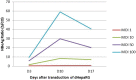
Currently, there is a lack of systems for studying the role of hepatitis B viral proteins, such as HBeAg and HBcAg, on liver injury. It is necessary to develop an original tool in order to clarify the role of these viral proteins in hepatic stellate cell activation, and to understand the molecular mechanisms of liver injury. HepaRG are the most reliable hepatocyte-like cells for studying liver functions or disorders. In this paper, we demonstrate that the transduction of differentiated HepaRG (dHepaRG) cells can be performed successfully using lentiviral particles. The production of a functional Green Fluorescent Protein (GFP) assessed by Fluorescence Activated Cell Sorting and fluorescence microscopy is up to 16% of GFP positive cells using a multiplicity of infection (MOI) of 2.4. We demonstrate that this technology can allow the stable expression of GFP during the long lifecycle of the cell (up to four weeks after the cell's passage). With this innovative tool, we aim to express viral proteins such as HBeAg or HBcAg in dHepaRG cells. The preliminary results of this work shows that HBeAg can be efficiently produced in dHepaRG cells and that increased MOI allows a better production of this protein. Our future objective will be to study the role of HBc and HBe proteins on the induction of hepatic fibrosis.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Yan, H. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife3 (2012). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

