Bipolenins K-N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana
- PMID: 31501669
- PMCID: PMC6720731
- DOI: 10.3762/bjoc.15.198
Bipolenins K-N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana
Abstract
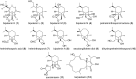
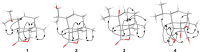
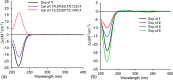
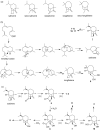
Chemical investigation of the barley and wheat fungal pathogen Bipolaris sorokiniana BRIP10943 yielded four new sativene-type sesquiterpenoid natural products, bipolenins K-N (1-4), together with seven related known analogues (5-11), and a sesterterpenoid (12). Their structures were determined by detailed analysis of spectroscopic data, supported by TDDFT calculations and comparison with previously reported analogues. These compounds were evaluated for their phytotoxic activity against wheat seedlings and wheat seed germination. The putative biosynthetic relationships between the isolated sesquiterpenoids were also explored.
Keywords: Bipolaris sorokiniana; phytotoxicity; sesquiterpenes; terpenes.
Figures
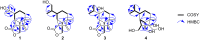
Similar articles
-
UPLC-Q-TOF-MS/MS Analysis of Seco-Sativene Sesquiterpenoids to Detect New and Bioactive Analogues From Plant Pathogen Bipolaris sorokiniana.Front Microbiol. 2022 Mar 9;13:807014. doi: 10.3389/fmicb.2022.807014. eCollection 2022. Front Microbiol. 2022. PMID: 35356527 Free PMC article.
-
Sativene-Related Sesquiterpenoids with Phytotoxic and Plant-Promoting Activities from the Plant Pathogenic Fungus Bipolaris sorokiniana Based on a Molecular Networking Strategy.J Agric Food Chem. 2025 Jan 8;73(1):562-570. doi: 10.1021/acs.jafc.4c09737. Epub 2024 Dec 27. J Agric Food Chem. 2025. PMID: 39729345
-
Seco-sativene and Seco-longifolene Sesquiterpenoids from Cultures of Endophytic Fungus Bipolaris eleusines.Nat Prod Bioprospect. 2017 Feb;7(1):147-150. doi: 10.1007/s13659-016-0116-4. Epub 2017 Jan 6. Nat Prod Bioprospect. 2017. PMID: 28063119 Free PMC article.
-
Bipolaris sorokiniana-Induced Black Point, Common Root Rot, and Spot Blotch Diseases of Wheat: A Review.Front Cell Infect Microbiol. 2021 Mar 11;11:584899. doi: 10.3389/fcimb.2021.584899. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33777829 Free PMC article. Review.
-
Naturally Occurring seco-Sativene Sesquiterpenoid: Chemistry and Biology.J Agric Food Chem. 2020 Sep 16;68(37):9827-9838. doi: 10.1021/acs.jafc.0c04560. Epub 2020 Sep 7. J Agric Food Chem. 2020. PMID: 32853522 Review.
Cited by
-
Survey, isolation and characterisation of Bipolaris sorokiniana (Shoem.) causing spot blotch disease in wheat under the climatic conditions of the Indo-Gangetic plains of India.Heliyon. 2024 Nov 14;10(22):e40398. doi: 10.1016/j.heliyon.2024.e40398. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39624279 Free PMC article.
-
UPLC-Q-TOF-MS/MS Analysis of Seco-Sativene Sesquiterpenoids to Detect New and Bioactive Analogues From Plant Pathogen Bipolaris sorokiniana.Front Microbiol. 2022 Mar 9;13:807014. doi: 10.3389/fmicb.2022.807014. eCollection 2022. Front Microbiol. 2022. PMID: 35356527 Free PMC article.
-
Production and identification of two antifungal terpenoids from the Posidonia oceanica epiphytic Ascomycota Mariannaea humicola IG100.Microb Cell Fact. 2020 Oct 1;19(1):184. doi: 10.1186/s12934-020-01445-7. Microb Cell Fact. 2020. PMID: 33004054 Free PMC article.
-
Differential Metabolomics Reveals Pathogenesis of Pestalotiopsis kenyana Causing Leaf Spot Disease of Zanthoxylum schinifolium.J Fungi (Basel). 2022 Nov 15;8(11):1208. doi: 10.3390/jof8111208. J Fungi (Basel). 2022. PMID: 36422029 Free PMC article.
-
New sesquiterpenoids with anti-inflammatory effects from phytopathogenic fungus Bipolaris sorokiniana 11134.Nat Prod Bioprospect. 2025 May 9;15(1):29. doi: 10.1007/s13659-025-00508-9. Nat Prod Bioprospect. 2025. PMID: 40343621 Free PMC article.
References
LinkOut - more resources
Full Text Sources
