Regulation of gap junction intercellular communication by connexin ubiquitination: physiological and pathophysiological implications
- PMID: 31501970
- PMCID: PMC7040059
- DOI: 10.1007/s00018-019-03285-0
Regulation of gap junction intercellular communication by connexin ubiquitination: physiological and pathophysiological implications
Abstract
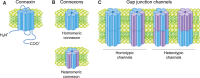
Gap junctions consist of arrays of intercellular channels that enable adjacent cells to communicate both electrically and metabolically. Gap junctions have a wide diversity of physiological functions, playing critical roles in both excitable and non-excitable tissues. Gap junction channels are formed by integral membrane proteins called connexins. Inherited or acquired alterations in connexins are associated with numerous diseases, including heart failure, neuropathologies, deafness, skin disorders, cataracts and cancer. Gap junctions are highly dynamic structures and by modulating the turnover rate of connexins, cells can rapidly alter the number of gap junction channels at the plasma membrane in response to extracellular or intracellular cues. Increasing evidence suggests that ubiquitination has important roles in the regulation of endoplasmic reticulum-associated degradation of connexins as well as in the modulation of gap junction endocytosis and post-endocytic sorting of connexins to lysosomes. In recent years, researchers have also started to provide insights into the physiological roles of connexin ubiquitination in specific tissue types. This review provides an overview of the advances made in understanding the roles of connexin ubiquitination in the regulation of gap junction intercellular communication and discusses the emerging physiological and pathophysiological implications of these processes.
Keywords: Cancer; Cataract; Electrical synapse; Heart arrhythmia; Heart ischemia; Lens; NEDD4; Ubiquitin.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

