Structural characterization of self-assembled chain like Fe-FeOx Core shell nanostructure
- PMID: 31502100
- PMCID: PMC6734011
- DOI: 10.1186/s11671-019-3128-2
Structural characterization of self-assembled chain like Fe-FeOx Core shell nanostructure
Abstract
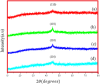
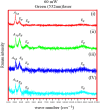
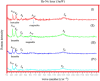
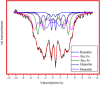
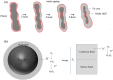
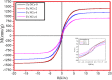
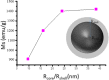
One of the big challenge of studying the core-shell iron nanostructures is to know the nature of oxide shell, i.e., whether it is γ-Fe2O3 (Maghemite), Fe3O4 (Magnetite), α-Fe2O3 (Hematite), or FeO (Wustite). By knowing the nature of iron oxide shell with zero valent iron core, one can determine the chemical or physical behavior of core-shell nanostructures. Fe core-shell nanochains (NCs) were prepared through the reduction of Fe3+ ions by sodium boro-hydride in aqueous solution at room atmosphere, and Fe NCs were further aged in water up to 240 min. XRD was used to study the structure of Fe NCs. Further analysis of core-shell nature of Fe NCs was done by TEM, results showed increase in thickness of oxide shell (from 2.5, 4, 6 to 10 nm) as water aging time increases (from 0 min, 120 min, 240 min to 360 min). The Raman spectroscopy was employed to study the oxide nature of Fe NCs. To further confirm the magnetite phase in Fe NCs, the Mössbauer spectroscopy was done on Fe NCs-0 and Fe NCs-6. Result shows the presence of magnetite in the sample before aging in water, and the sample after prolonged aging contains pure Hematite phase. It shows that prolonged water oxidation transforms the structure of shell of Fe NCs from mixture of Hematite and Magnetite in to pure hematite shell. The Magnetic properties of the Fe NCs were measured by VSM at 320 K. Because of high saturation magnetization (Ms) values, Fe NCs could be used as r2 contrasts agents for magnetic resonance imaging (MRI) in near future.
Keywords: 1-D nanostructures; Fe nanochains; Raman spectroscopy; Transmission electron microscopy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Synthesis of superparamagnetic bare Fe₃O₄ nanostructures and core/shell (Fe₃O₄/alginate) nanocomposites.Carbohydr Polym. 2012 Jul 1;89(3):821-9. doi: 10.1016/j.carbpol.2012.04.016. Epub 2012 Apr 13. Carbohydr Polym. 2012. PMID: 24750867
-
Bifunctional nanoscale magnetic chains with high saturation magnetization and catalytic activity.J Colloid Interface Sci. 2018 Sep 1;525:152-160. doi: 10.1016/j.jcis.2018.04.080. Epub 2018 Apr 21. J Colloid Interface Sci. 2018. PMID: 29702321
-
Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water.Chemosphere. 2015 Jan;119:1068-1074. doi: 10.1016/j.chemosphere.2014.09.026. Epub 2014 Oct 14. Chemosphere. 2015. PMID: 25317915
-
Current Status of Magnetite-Based Core@Shell Structures for Diagnosis and Therapy in Oncology Short running title: Biomedical Applications of Magnetite@Shell Structures.Curr Pharm Des. 2015;21(37):5417-33. doi: 10.2174/1381612821666150917093543. Curr Pharm Des. 2015. PMID: 26377654 Review.
-
Mössbauer spectroscopic investigations on iron oxides and modified nanostructures: A review.J Mater Res. 2023;38(4):937-957. doi: 10.1557/s43578-022-00665-4. Epub 2022 Aug 29. J Mater Res. 2023. PMID: 36059887 Free PMC article. Review.
Cited by
-
Multi-Segmented Nanowires: A High Tech Bright Future.Materials (Basel). 2019 Nov 26;12(23):3908. doi: 10.3390/ma12233908. Materials (Basel). 2019. PMID: 31779229 Free PMC article. Review.
-
From Microenvironment Remediation to Novel Anti-Cancer Strategy: The Emergence of Zero Valent Iron Nanoparticles.Pharmaceutics. 2022 Jan 2;14(1):99. doi: 10.3390/pharmaceutics14010099. Pharmaceutics. 2022. PMID: 35056996 Free PMC article. Review.
-
Lessons from Nature: Advances and Perspectives in Bionic Microwave Absorption Materials.Nanomicro Lett. 2024 Dec 30;17(1):100. doi: 10.1007/s40820-024-01591-2. Nanomicro Lett. 2024. PMID: 39739207 Free PMC article. Review.
References
-
- Lin W-S, Lin H-M, Chen H-H, Hwu Y-K, Chiou Y-J. Shape effects of iron nanowires on hyperthermia treatment. J Nanomater. 2013;2013:6.
LinkOut - more resources
Full Text Sources

