A convenient and efficient total solid-phase synthesis of DOTA-functionalized tumor-targeting peptides for PET imaging of cancer
- PMID: 31502101
- PMCID: PMC6733935
- DOI: 10.1186/s13550-019-0539-0
A convenient and efficient total solid-phase synthesis of DOTA-functionalized tumor-targeting peptides for PET imaging of cancer
Abstract
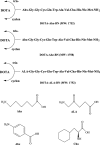
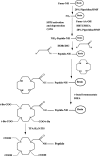
Introduction: An efficient and cost-effective synthesis of the metal chelating agents that couple to tumor-targeting peptides is required to enhance the process of preclinical research toward the clinical translation of molecular imaging agents. DOTA is one of the most widely used macrocyclic ligands for the development of new metal-based imaging and therapeutic agents owing to its ability to form stable and inert complexes under physiological conditions. Although solid-phase synthesis compatible DOTA-tris-(t-Bu ester) is a commercial product, it is expensive and contain chemical impurities. There is a need to explore new and cost-effective methods for the preparation of metal chelating agents, i.e., DOTA, directly on solid support to facilitate rapid, cost-effective, and high purity preparation of DOTA-linked peptides for imaging and therapy. In the present study, we describe a facile synthetic strategy of DOTA preparation and its linkage to peptides directly on solid-phase support.
Methods: Bombesin (BN) peptides were functionalized with DOTA chelator prepared from cyclen precursor on solid-phase and from commercial DOTA-tris and radiolabeled with 68Ga. In vitro BN/GRP receptor binding affinities of the corresponding radiolabeled peptides were determined by saturation binding assays on human breast MDA-MB-231, MCF7, T47D, and PC3 prostate cancer cells. Pharmacokinetics were studied in Balb/c mice and in vivo tumor targeting in MDA-MB-231 tumor-bearing nude mice.
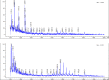
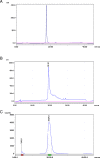
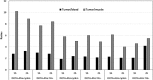
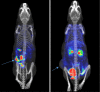
Results: DOTA was prepared successfully from cyclen on solid-phase support, linked specifically to BN peptides and resultant DOTA-coupled peptides were radiolabeled efficiently with 68Ga. The binding affinities of all the six BN peptides were comparable and in the low nanomolar range. All 68Ga-labeled peptides showed high metabolic stability in plasma. These radiopeptides exhibited rapid pharmacokinetics in Balb/c mice with excretion mainly through the urinary system. In nude mice, MDA-MB-231 tumor uptake profiles were slightly different; the BN peptide with Ahx spacer and linked to DOTA through cyclen exhibited higher tumor uptake (2.32% ID/g at 1 h post-injection) than other radiolabeled BN peptides investigated in this study. The same leading BN peptide also displayed favorable pharmacokinetic profile in Balb/c mice. The PET images clearly visualized the MDA-MB-231 tumor.
Conclusions: DOTA prepared from cyclen on solid-phase support showed comparable potency and efficiency to DOTA-tris in both in vitro and in vivo evaluation. The synthetic methodology described here allows versatile, site-specific introduction of DOTA into peptides to facilitate the development of DOTA-linked molecular imaging and therapy agents for clinical translation.
Keywords: Biodistribution; Bombesin; DOTA; Solid-phase peptide synthesis; Tumor imaging; cyclen.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
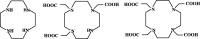
Similar articles
-
Preparation, Radiolabeling with 68Ga/177Lu and Preclinical Evaluation of Novel Angiotensin Peptide Analog: A New Class of Peptides for Breast Cancer Targeting.Pharmaceuticals (Basel). 2023 Nov 2;16(11):1550. doi: 10.3390/ph16111550. Pharmaceuticals (Basel). 2023. PMID: 38004416 Free PMC article.
-
Synthesis and evaluation of a technetium-99m labeled cytotoxic bombesin peptide conjugate for targeting bombesin receptor-expressing tumors.Nucl Med Biol. 2010 Apr;37(3):277-88. doi: 10.1016/j.nucmedbio.2009.12.006. Epub 2010 Feb 10. Nucl Med Biol. 2010. PMID: 20346867
-
Preparation and evaluation of bombesin peptide derivatives as potential tumor imaging agents: effects of structure and composition of amino acid sequence on in vitro and in vivo characteristics.Nucl Med Biol. 2012 Aug;39(6):795-804. doi: 10.1016/j.nucmedbio.2012.01.002. Epub 2012 Mar 3. Nucl Med Biol. 2012. PMID: 22381782
-
Peptide-based imaging agents for cancer detection.Adv Drug Deliv Rev. 2017 Feb;110-111:38-51. doi: 10.1016/j.addr.2016.06.007. Epub 2016 Jun 18. Adv Drug Deliv Rev. 2017. PMID: 27327937 Free PMC article. Review.
-
Peptide PET Imaging: A Review of Recent Developments and a Look at the Future of Radiometal-Labeled Peptides in Medicine.Chem Biomed Imaging. 2024 Aug 23;2(9):615-630. doi: 10.1021/cbmi.4c00030. eCollection 2024 Sep 23. Chem Biomed Imaging. 2024. PMID: 39474267 Free PMC article. Review.
Cited by
-
Preparation and preclinical evaluation of 18F-labeled folate-RGD peptide conjugate for PET imaging of triple-negative breast carcinoma.EJNMMI Radiopharm Chem. 2025 May 19;10(1):25. doi: 10.1186/s41181-025-00349-4. EJNMMI Radiopharm Chem. 2025. PMID: 40389652 Free PMC article.
-
Preparation, Radiolabeling with 68Ga/177Lu and Preclinical Evaluation of Novel Angiotensin Peptide Analog: A New Class of Peptides for Breast Cancer Targeting.Pharmaceuticals (Basel). 2023 Nov 2;16(11):1550. doi: 10.3390/ph16111550. Pharmaceuticals (Basel). 2023. PMID: 38004416 Free PMC article.
-
Peptide-Functionalized Nanomedicine: Advancements in Drug Delivery, Diagnostics, and Biomedical Applications.Molecules. 2025 Mar 31;30(7):1572. doi: 10.3390/molecules30071572. Molecules. 2025. PMID: 40286158 Free PMC article. Review.
-
Advances in Development of Radiometal Labeled Amino Acid-Based Compounds for Cancer Imaging and Diagnostics.Pharmaceuticals (Basel). 2021 Feb 21;14(2):167. doi: 10.3390/ph14020167. Pharmaceuticals (Basel). 2021. PMID: 33669938 Free PMC article. Review.
-
Radiometallation and photo-triggered release of ready-to-inject radiopharmaceuticals from the solid phase.Chem Sci. 2023 Apr 17;14(19):5038-5050. doi: 10.1039/d2sc06977f. eCollection 2023 May 17. Chem Sci. 2023. PMID: 37206398 Free PMC article.
References
-
- Chappell LL, Rogers BE, Khazaeli MB, Mayo MS, Buchsbaum DJ, Brechbiel MW. Improved synthesis of the bifunctional chelating agent 1,4,7,10-Tetraaza-N-(1-carboxy-3-(4-nitrophenyl)propyl)-N′,N′′,N′′′-tris(acetic acid)cyclododecane (PA-DOTA) Bioorg Med Chem. 1999;7:2313–2320. doi: 10.1016/S0968-0896(99)00171-6. - DOI - PubMed
-
- Cakic N, Gündüz S, Rengarasu R, Angelovski G. Synthetic strategies for preparation of cyclen-based MRI contrast agents. Tetrahedron Letters. 2015;56:759–765. doi: 10.1016/j.tetlet.2014.12.087. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

