CE-MS with electrokinetic supercharging and application to determination of neurotransmitters
- PMID: 31502303
- PMCID: PMC6947659
- DOI: 10.1002/elps.201900203
CE-MS with electrokinetic supercharging and application to determination of neurotransmitters
Abstract
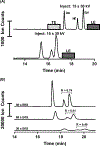
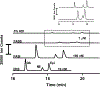
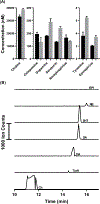
Electrokinetic supercharging (EKS) is known as one of the most effective online electrophoretic preconcentration techniques, though pairing with it with mass spectrometry has presented challenges. Here, EKS is successfully paired with ESI-MS/MS to provide a sensitive and robust method for analysis of biogenic amines in biological samples. Injection parameters including electric field strength and the buffer compositions used for the separation and focusing were investigated to achieve suitable resolution, high sensitivity, and compatibility with ESI-MS. Using EKS, the sensitivity of the method was improved 5000-fold compared to a conventional hydrodynamic injection with CZE. The separation allowed for baseline resolution of several neurotransmitters within 16 min with LODs down to 10 pM. This method was applied to targeted analysis of seven biogenic amines from rat brain stem and whole Drosophila tissue. This is the first method to use EKS with CE-ESI-MS/MS to analyze biological samples.
Keywords: Biological samples; CE; CE-MS; Electrokinetic supercharging; Neurotransmitters.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
Electrokinetic supercharging-electrospray ionisation-mass spectrometry for separation and on-line preconcentration of hypolipidaemic drugs in water samples.Electrophoresis. 2010 Apr;31(7):1184-1193. doi: 10.1002/elps.200900420. Electrophoresis. 2010. PMID: 20349512
-
Online preconcentration by electrokinetic supercharging for separation of endocrine disrupting chemical and phenolic pollutants in water samples.Electrophoresis. 2016 Oct;37(20):2649-2656. doi: 10.1002/elps.201600207. Epub 2016 Aug 23. Electrophoresis. 2016. PMID: 27434368
-
Integration of the free liquid membrane into electrokinetic supercharging - capillary electrophoresis for the determination of cationic herbicides in environmental water samples.J Chromatogr A. 2017 Jan 20;1481:145-151. doi: 10.1016/j.chroma.2016.12.042. Epub 2016 Dec 15. J Chromatogr A. 2017. PMID: 28017568
-
[Advances in chiral separation and analysis by capillary electrophoresis-mass spectrometry].Se Pu. 2022 Jun;40(6):509-519. doi: 10.3724/SP.J.1123.2021.11006. Se Pu. 2022. PMID: 35616196 Free PMC article. Review. Chinese.
-
[Capillary electrophoresis-mass spectrometry and its application to proteomic analysis].Se Pu. 2020 Oct 8;38(10):1117-1124. doi: 10.3724/SP.J.1123.2020.03005. Se Pu. 2020. PMID: 34213108 Review. Chinese.
Cited by
-
Recent advances (2019-2021) of capillary electrophoresis-mass spectrometry for multilevel proteomics.Mass Spectrom Rev. 2023 Mar;42(2):617-642. doi: 10.1002/mas.21714. Epub 2021 Jun 15. Mass Spectrom Rev. 2023. PMID: 34128246 Free PMC article. Review.
-
CE-MS for metabolomics: Developments and applications in the period 2018-2020.Electrophoresis. 2021 Feb;42(4):381-401. doi: 10.1002/elps.202000203. Epub 2020 Oct 4. Electrophoresis. 2021. PMID: 32906195 Free PMC article. Review.
-
Recent (2018-2020) development in capillary electrophoresis.Anal Bioanal Chem. 2022 Jan;414(1):115-130. doi: 10.1007/s00216-021-03290-y. Epub 2021 Mar 22. Anal Bioanal Chem. 2022. PMID: 33754195 Free PMC article. Review.
-
A Hypothesis From Metabolomics Analysis of Diabetic Retinopathy: Arginine-Creatine Metabolic Pathway May Be a New Treatment Strategy for Diabetic Retinopathy.Front Endocrinol (Lausanne). 2022 Mar 24;13:858012. doi: 10.3389/fendo.2022.858012. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35399942 Free PMC article. Review.
References
-
- Chen G, Ewing AG Crit Rev Neurobiol. 1997, 11, 59. - PubMed
-
- Lapainis T, Sweedler JV, J Chromatogr A 2008, 1184, 144–158. - PubMed
-
- Monton MRN, Soga T, Chromatogr J. A 2007, 1168, 237–246. - PubMed
-
- Faserl K, Sarg B, Sola L, Lindner HH, Proteomics 2017, 17, 1–5. - PubMed
-
- Mateos-Vivas M, Domínguez-Álvarez J, Rodríguez-Gonzalo E, Carabias-Martínez R, Food Chem. 2017, 233, 38–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

