Mechanistic Investigation of Enantioconvergent Kumada Reactions of Racemic α-Bromoketones Catalyzed by a Nickel/Bis(oxazoline) Complex
- PMID: 31502449
- PMCID: PMC7075318
- DOI: 10.1021/jacs.9b08185
Mechanistic Investigation of Enantioconvergent Kumada Reactions of Racemic α-Bromoketones Catalyzed by a Nickel/Bis(oxazoline) Complex
Abstract
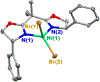
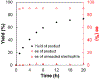
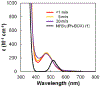
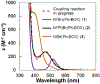
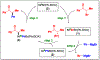
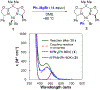
In recent years, a wide array of methods for achieving nickel-catalyzed substitution reactions of alkyl electrophiles by organometallic nucleophiles, including enantioconvergent processes, have been described; however, experiment-focused mechanistic studies of such couplings have been comparatively scarce. The most detailed mechanistic investigations to date have examined catalysts that bear tridentate ligands and, with one exception, processes that are not enantioselective; studies of catalysts based on bidentate ligands could be anticipated to be more challenging, due to difficulty in isolating proposed intermediates as a result of instability arising from coordinative unsaturation. In this investigation, we explore the mechanism of enantioconvergent Kumada reactions of racemic α-bromoketones catalyzed by a nickel complex that bears a bidentate chiral bis(oxazoline) ligand. Utilizing an array of mechanistic tools (including isolation and reactivity studies of three of the four proposed nickel-containing intermediates, as well as interrogation via EPR spectroscopy, UV-vis spectroscopy, radical probes, and DFT calculations), we provide support for a pathway in which carbon-carbon bond formation proceeds via a radical-chain process wherein a nickel(I) complex serves as the chain-carrying radical and an organonickel(II) complex is the predominant resting state of the catalyst. Computations indicate that the coupling of this organonickel(II) complex with an organic radical is the stereochemistry-determining step of the reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
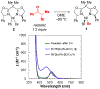
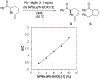

Similar articles
-
Nickel/bis(oxazoline)-catalyzed asymmetric Kumada reactions of alkyl electrophiles: cross-couplings of racemic alpha-bromoketones.J Am Chem Soc. 2010 Feb 3;132(4):1264-6. doi: 10.1021/ja909689t. J Am Chem Soc. 2010. PMID: 20050651 Free PMC article.
-
Enantioconvergent Cross-Couplings of Alkyl Electrophiles: The Catalytic Asymmetric Synthesis of Organosilanes.Angew Chem Int Ed Engl. 2019 Mar 11;58(11):3571-3574. doi: 10.1002/anie.201814208. Epub 2019 Feb 6. Angew Chem Int Ed Engl. 2019. PMID: 30650228 Free PMC article.
-
Catalytic enantioselective cross-couplings of secondary alkyl electrophiles with secondary alkylmetal nucleophiles: Negishi reactions of racemic benzylic bromides with achiral alkylzinc reagents.J Am Chem Soc. 2012 Oct 17;134(41):17003-6. doi: 10.1021/ja308460z. Epub 2012 Oct 5. J Am Chem Soc. 2012. PMID: 23039358 Free PMC article.
-
Ni-Catalyzed C-C Couplings Using Alkyl Electrophiles.Top Curr Chem (Cham). 2016 Oct;374(5):66. doi: 10.1007/s41061-016-0067-6. Epub 2016 Aug 31. Top Curr Chem (Cham). 2016. PMID: 27580894 Review.
-
Ligand Development for Copper-Catalyzed Enantioconvergent Radical Cross-Coupling of Racemic Alkyl Halides.J Am Chem Soc. 2022 Sep 28;144(38):17319-17329. doi: 10.1021/jacs.2c06718. Epub 2022 Sep 1. J Am Chem Soc. 2022. PMID: 36048164 Review.
Cited by
-
Nickel-Catalyzed α-Carbonylalkylarylation of Vinylarenes: Expedient Access to γ,γ-Diarylcarbonyl and Aryltetralone Derivatives.Angew Chem Int Ed Engl. 2020 May 18;59(21):8047-8051. doi: 10.1002/anie.201913435. Epub 2020 Mar 19. Angew Chem Int Ed Engl. 2020. PMID: 32059062 Free PMC article.
-
A Widely Applicable Dual Catalytic System for Cross-Electrophile Coupling Enabled by Mechanistic Studies.ACS Catal. 2020 Nov 6;10(21):12642-12656. doi: 10.1021/acscatal.0c03237. Epub 2020 Sep 29. ACS Catal. 2020. PMID: 33628617 Free PMC article.
-
Enantioselective α-Arylation of Ketones via a Novel Cu(I)-Bis(phosphine) Dioxide Catalytic System.J Am Chem Soc. 2021 Mar 10;143(9):3289-3294. doi: 10.1021/jacs.0c13236. Epub 2021 Feb 26. J Am Chem Soc. 2021. PMID: 33635068 Free PMC article.
-
Metal-Catalyzed Enantioconvergent Transformations.Chem Rev. 2023 Oct 25;123(20):11817-11893. doi: 10.1021/acs.chemrev.3c00059. Epub 2023 Oct 4. Chem Rev. 2023. PMID: 37793021 Free PMC article. Review.
-
General Method for Enantioselective Three-Component Carboarylation of Alkenes Enabled by Visible-Light Dual Photoredox/Nickel Catalysis.J Am Chem Soc. 2020 Nov 19:10.1021/jacs.0c08823. doi: 10.1021/jacs.0c08823. Online ahead of print. J Am Chem Soc. 2020. PMID: 33211954 Free PMC article.
References
-
- Choi J; Fu GC Transition Metal-Catalyzed Alkyl–Alkyl Bond Formation: Another Dimension in Cross-Coupling Chemistry. Science 2017, 356, eaaf7230. - PMC - PubMed
- Fu GC Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Cent. Sci 2017, 3, 692–700. - PMC - PubMed
- Kaga A; Chiba S Engaging Radicals in Transition Metal-Catalyzed Cross-Coupling with Alkyl Electrophiles: Recent Advances. ACS Catal. 2017, 7, 4697–4706.
- Iwasaki T; Kambe N Ni-Catalyzed C–C Couplings using Alkyl Electrophiles. Top. Curr. Chem 2016, 374, 66. - PubMed
-
-
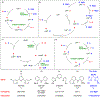
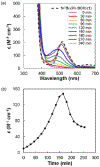
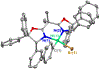
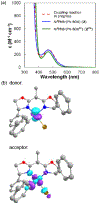
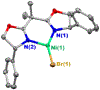
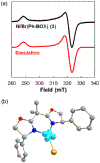
Figure 1 summarizes experiment-focused mechanistic studies wherein at least one proposed organonickel intermediate has been characterized:
- Jones GD; Martin JL; McFarland C; Allen OR; Hall RE; Haley AD; Brandon RJ; Konovalova T; Desrochers PJ; Pulay P; Vicic DA Ligand Redox Effects in the Synthesis, Electronic Structure, and Reactivity of an Alkyl-Alkyl Cross-Coupling Catalyst. J. Am. Chem. Soc 2006, 128, 13175−13183. - PubMed
- Breitenfeld J; Ruiz J; Wodrich MD; Hu X Bimetallic Oxidative Addition Involving Radical Intermediates in Nickel-Catalyzed Alkyl–Alkyl Kumada Coupling Reactions. J. Am. Chem. Soc 2013, 135, 12004–12012; Breitenfeld J; Wodrich MD; Hu X Bimetallic Oxidative Addition in Nickel-Catalyzed Alkyl–Aryl Kumada Coupling Reactions. Organometallics 2014, 33, 5708–5715. - PubMed
- Schley ND; Fu GC Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation. J. Am. Chem. Soc 2014, 136, 16588–16593. - PMC - PubMed
- Patel UN; Jain S; Pandey DK; Gonnade RG; Vanka K; Punji B Mechanistic Aspects of Pincer Nickel(II)-Catalyzed C–H Bond Alkylation of Azoles with Alkyl Halides. Organometallics 2018, 37, 1017–1025.
-
-
-
For examples of computation-based mechanistic studies, see:
- Lin X; Phillips DL Density Functional Theory Studies of Negishi Alkyl–Alkyl Cross-Coupling Reactions Catalyzed by a Methylterpyridyl-Ni(I) Complex. J. Org. Chem 2008, 73, 3680–3688. - PubMed
- Chass GA; Kantchev EAB; Fang D-C The Fine Balance Between One Cross-Coupling and Two β-Hydride Elimination Pathways: A DFT Mechanistic Study of Ni(π-allyl)2-Catalyzed Cross-Coupling of Alkyl Halides and Alkyl Grignard Reagents. Chem. Commun 2010, 46, 2727–2729. - PubMed
- Lin X; Sun J; Xi Y; Lin D How Racemic Secondary Alkyl Electrophiles Proceed to Enantioselective Products in Negishi Cross-Coupling Reactions. Organometallics 2011, 30, 3284–3292.
- Li Z; Jiang Y-Y; Fu Y Theoretical Study on the Mechanism of Ni-Catalyzed Alkyl–Alkyl Suzuki Cross-Coupling. Chem. Eur. J 2012, 18, 4345–4357. - PubMed
- Gutierrez O; Tellis JC; Primer DN; Molander GA; Kozlowski MC Nickel-Catalyzed Cross-Coupling of Photoredox-Generated Radicals: Uncovering a General Manifold for Stereoconvergence in Nickel-Catalyzed Cross-Couplings. J. Am. Chem. Soc 2015, 137, 4896–4899. - PMC - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

