Incorporating Ontogeny in Physiologically Based Pharmacokinetic Modeling to Improve Pediatric Drug Development: What We Know About Developmental Changes in Membrane Transporters
- PMID: 31502692
- PMCID: PMC7408403
- DOI: 10.1002/jcph.1489
Incorporating Ontogeny in Physiologically Based Pharmacokinetic Modeling to Improve Pediatric Drug Development: What We Know About Developmental Changes in Membrane Transporters
Abstract
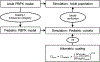
Developmental changes in the biological processes involved in the disposition of drugs, such as membrane transporter expression and activity, may alter the drug exposure and clearance in pediatric patients. Physiologically based pharmacokinetic (PBPK) models take these age-dependent changes into account and may be used to predict drug exposure in children. As a result, this mechanistic-based tool has increasingly been applied to improve pediatric drug development. Under the Prescription Drug User Fee Act VI, the US Food and Drug Administration has committed to facilitate the advancement of PBPK modeling in the drug application review process. Yet, significant knowledge gaps on developmental biology still exist, which must be addressed to increase the confidence of prediction. Recently, more data on ontogeny of transporters have emerged and supplied a missing piece of the puzzle. This article highlights the recent findings on the ontogeny of transporters specifically in the intestine, liver, and kidney. It also provides a case study that illustrates the utility of incorporating this information in predicting drug exposure in children using a PBPK approach. Collaborative work has greatly improved the understanding of the interplay between developmental physiology and drug disposition. Such efforts will continue to be needed to address the remaining knowledge gaps to enhance the application of PBPK modeling in drug development for children.
Keywords: PBPK; children; model-informed drug development; ontogeny; pediatric; physiologically based pharmacokinetic modeling; transporters.
© 2019, The American College of Clinical Pharmacology.
Conflict of interest statement
Conflicts of Interest
The authors declare no conflicts of interest for this work.
Figures
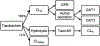

References
-
- Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Addressing the barriers to pediatric drug development: workshop summary In: The National Academies Collection: Reports Funded by National Institutes of Health. Washington, DC: National Academies Press; 2008. - PubMed
-
- Food US and Administration Drug. Best Pharmaceuticals for Children Act and Pediatric Research Equity Act. https://www.da.gov/science-research/pediatrics/best-pharmaceuticals-chil... Accessed May 16, 2019.
-
- European Medicines Agency. Paediatric Regulation. https://www.ema.europa.eu/en/human-regulatory/overview/paediatric-medici.... Accessed June 13, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

