Beyond Tethering the Viral Particles: Immunomodulatory Functions of Tetherin (BST-2)
- PMID: 31502877
- PMCID: PMC6939588
- DOI: 10.1089/dna.2019.4777
Beyond Tethering the Viral Particles: Immunomodulatory Functions of Tetherin (BST-2)
Abstract
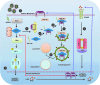
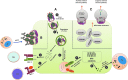
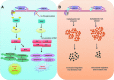
Host response to viral infection is a highly regulated process involving engagement of various host factors, cytokines, chemokines, and stimulatory signals that pave the way for an antiviral immune response. The response is manifested in terms of viral sequestration, phagocytosis, and inhibition of genome replication, and, finally, if required, lymphocyte-mediated clearance of virally infected cells. During this process, cross-talk between viral and host factors can shape disease outcomes and immunopathology. Bone marrow stromal antigen 2 (BST-2), also know as tetherin, is induced by type I interferon produced in response to viral infections, as well as in certain cancers. BST-2 has been shown to be a host restriction factor of virus multiplication through its ability to physically tether budding virions and restrict viral spread. However, BST-2 has other roles in the host antiviral response. This review focuses on the diverse functions of BST-2 and its downstream signaling pathways in regulating host immune responses.
Keywords: antiviral; cancer; immunomodulatory; tetherin.
Conflict of interest statement
No competing financial interest exists.
Figures
Similar articles
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
-
Limiting Respiratory Viral Infection by Targeting Antiviral and Immunological Functions of BST-2/Tetherin: Knowledge and Gaps.Bioessays. 2018 Oct;40(10):e1800086. doi: 10.1002/bies.201800086. Epub 2018 Aug 16. Bioessays. 2018. PMID: 30113067 Free PMC article. Review.
-
Severe Acute Respiratory Syndrome Coronavirus ORF7a Inhibits Bone Marrow Stromal Antigen 2 Virion Tethering through a Novel Mechanism of Glycosylation Interference.J Virol. 2015 Dec;89(23):11820-33. doi: 10.1128/JVI.02274-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378163 Free PMC article.
-
TLR-4 engagement of dendritic cells confers a BST-2/tetherin-mediated restriction of HIV-1 infection to CD4+ T cells across the virological synapse.Retrovirology. 2013 Jan 11;10:6. doi: 10.1186/1742-4690-10-6. Retrovirology. 2013. PMID: 23311681 Free PMC article.
-
An unconventional BST-2 function: down-regulation of transient protein expression.Biochem Biophys Res Commun. 2014 Aug 8;450(4):1469-74. doi: 10.1016/j.bbrc.2014.07.022. Epub 2014 Jul 11. Biochem Biophys Res Commun. 2014. PMID: 25019996
Cited by
-
Plasmacytoid dendritic cells at the forefront of anti-cancer immunity: rewiring strategies for tumor microenvironment remodeling.J Exp Clin Cancer Res. 2024 Jul 17;43(1):196. doi: 10.1186/s13046-024-03121-9. J Exp Clin Cancer Res. 2024. PMID: 39020402 Free PMC article. Review.
-
Uncovering transcriptional dark matter via gene annotation independent single-cell RNA sequencing analysis.Nat Commun. 2021 Apr 12;12(1):2158. doi: 10.1038/s41467-021-22496-3. Nat Commun. 2021. PMID: 33846360 Free PMC article.
-
Plasmacytoid Dendritic Cells as a Novel Cell-Based Cancer Immunotherapy.Int J Mol Sci. 2022 Sep 27;23(19):11397. doi: 10.3390/ijms231911397. Int J Mol Sci. 2022. PMID: 36232698 Free PMC article. Review.
-
TLR-mediated aggresome-like induced structures comprise antimicrobial peptides and attenuate intracellular bacterial survival.Mol Biol Cell. 2024 Mar 1;35(3):ar34. doi: 10.1091/mbc.E23-09-0347. Epub 2024 Jan 3. Mol Biol Cell. 2024. PMID: 38170582 Free PMC article.
-
Molecular intersections of traumatic brain injury and Alzheimer's disease: the role of ADMSC-derived exosomes and hub genes in microglial polarization.Metab Brain Dis. 2024 Dec 24;40(1):77. doi: 10.1007/s11011-024-01503-8. Metab Brain Dis. 2024. PMID: 39715972
References
-
- Brown D., Trowsdale J., and Allen R. (2004). The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 64, 215–225 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

