Reactive oxygen species-mediated senescence is accelerated by inhibiting Cdk2 in Idh2-deficient conditions
- PMID: 31503005
- PMCID: PMC6756887
- DOI: 10.18632/aging.102259
Reactive oxygen species-mediated senescence is accelerated by inhibiting Cdk2 in Idh2-deficient conditions
Abstract
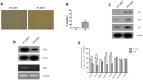
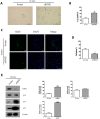
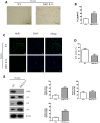
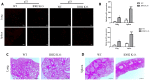
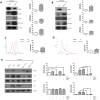
Among the many factors that promote cellular senescence, reactive oxygen species (ROS) are a focus of intense research because of their critical role in accelerating cellular senescence and initiating senescence-related diseases that can be fatal. Therefore, maintaining the proper balance of ROS in cells is a key method to alleviate senescence. Recent studies have found that isocitrate dehydrogenase 2 (IDH2), a critical enzyme of the tricarboxylic acid cycle, participates in ROS generation and in cellular dysfunction that is induced by excessive levels of ROS. Loss of IDH2 induces mitochondrial dysfunction that promotes excessive ROS generation and the development of several diseases. The results of this study suggest that Idh2 plays an important role in cellular senescence. Idh2 deficiency resulted in senescence-associated phenotypes and increased levels of senescence marker proteins in mouse embryonic fibroblasts and tissues. Furthermore, excessive ROS were generated in Idh2-deficient conditions, promoting cellular senescence by inducing cell cycle arrest through cyclin-dependent kinase 2. These results indicate that loss of Idh2 is a critical factor in regulating cellular senescence. Taken together, our findings contribute to the field of senescence research and suggest that IDH2 is a potential target of future anti-senescence studies.
Keywords: cell cycle; cyclin-dependent kinase 2 (Cdk2); isocitrate dehydrogenase 2 (IDH2); reactive oxygen species (ROS); senescence.
Conflict of interest statement
Figures


Similar articles
-
IDH2: A novel biomarker for environmental exposure in blood circulatory system disorders.Oncol Lett. 2022 Jun 24;24(2):278. doi: 10.3892/ol.2022.13398. eCollection 2022 Aug. Oncol Lett. 2022. PMID: 35814829 Free PMC article. Review.
-
IDH2 deficiency promotes mitochondrial dysfunction and cardiac hypertrophy in mice.Free Radic Biol Med. 2015 Mar;80:84-92. doi: 10.1016/j.freeradbiomed.2014.12.018. Epub 2014 Dec 31. Free Radic Biol Med. 2015. PMID: 25557279
-
Isocitrate dehydrogenase 2 deficiency induces endothelial inflammation via p66sh-mediated mitochondrial oxidative stress.Biochem Biophys Res Commun. 2018 Sep 10;503(3):1805-1811. doi: 10.1016/j.bbrc.2018.07.117. Epub 2018 Jul 30. Biochem Biophys Res Commun. 2018. PMID: 30072100
-
Therapeutic potential of the mitochondria-targeted antioxidant MitoQ in mitochondrial-ROS induced sensorineural hearing loss caused by Idh2 deficiency.Redox Biol. 2019 Jan;20:544-555. doi: 10.1016/j.redox.2018.11.013. Epub 2018 Nov 20. Redox Biol. 2019. PMID: 30508699 Free PMC article.
-
Free radicals and senescence.Exp Cell Res. 2008 Jun 10;314(9):1918-22. doi: 10.1016/j.yexcr.2008.01.011. Epub 2008 Jan 26. Exp Cell Res. 2008. PMID: 18282568 Free PMC article. Review.
Cited by
-
Extracellular citrate and metabolic adaptations of cancer cells.Cancer Metastasis Rev. 2021 Dec;40(4):1073-1091. doi: 10.1007/s10555-021-10007-1. Epub 2021 Dec 21. Cancer Metastasis Rev. 2021. PMID: 34932167 Free PMC article. Review.
-
IDH2: A novel biomarker for environmental exposure in blood circulatory system disorders.Oncol Lett. 2022 Jun 24;24(2):278. doi: 10.3892/ol.2022.13398. eCollection 2022 Aug. Oncol Lett. 2022. PMID: 35814829 Free PMC article. Review.
-
Reciprocal REGγ-Nrf2 Regulation Promotes Long Period ROS Scavenging in Oxidative Stress-Induced Cell Aging.Oxid Med Cell Longev. 2023 Jan 10;2023:4743885. doi: 10.1155/2023/4743885. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36659906 Free PMC article.
-
lncRNA OTUD6B-AS1 Exacerbates As2O3-Induced Oxidative Damage in Bladder Cancer via miR-6734-5p-Mediated Functional Inhibition of IDH2.Oxid Med Cell Longev. 2020 Sep 1;2020:3035624. doi: 10.1155/2020/3035624. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32952848 Free PMC article.
-
The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis.Cell Mol Life Sci. 2020 Oct;77(19):3729-3743. doi: 10.1007/s00018-020-03497-9. Epub 2020 May 28. Cell Mol Life Sci. 2020. PMID: 32468094 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

