Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression
- PMID: 31503404
- PMCID: PMC7064962
- DOI: 10.1111/1753-0407.12986
Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression
Abstract
Background: Impaired intestinal barrier structure and function have been validated as an important pathogenic process in type 2 diabetes mellitus (T2DM). Gut dysbiosis is thought to be the critical factor in diabetic intestinal pathogenesis. As the most abundant commensal bacteria, Faecalibacterium prausnitzii (F. prausnitzii) play important roles in gut homeostasis. The microbial anti-inflammatory molecule (MAM), an F. prausnitzii metabolite, has anti-inflammatory potential in inflammatory bowel disease (IBD). Thus, we aimed to explore the function and mechanism of MAM on the diabetic intestinal epithelium.
Methods: 16S high-throughput sequencing was used to analyze the gut microbiota of db/db mice (T2DM mouse model). We transfected a FLAG-tagged MAM plasmid into human colonic cells to explore the protein-protein interactions and observe cell monolayer permeability. For in vivo experiments, db/db mice were supplemented with recombinant His-tagged MAM protein from E. coli BL21 (DE3).
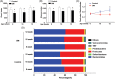
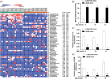
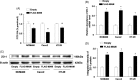
Results: The abundance of F. prausnitzii was downregulated in the gut microbiota of db/db mice. Immunoprecipitation (IP) and mass spectroscopy (MS) analyses revealed that MAM potentially interacts with proteins in the tight junction pathway, including zona occludens 1 (ZO-1). FLAG-tagged MAM plasmid transfection stabilized the cell permeability and increased ZO-1 expression in NCM460, Caco2, and HT-29 cells. The db/db mice supplemented with recombinant His-tagged MAM protein showed restored intestinal barrier function and elevated ZO-1 expression.
Conclusions: Our study shows that MAM from F. prausnitzii can restore the intestinal barrier structure and function in DM conditions via the regulation of the tight junction pathway and ZO-1 expression.
背景: 肠道屏障结构和功能异常是 2 型糖尿病的重要病理改变之一。 而肠道菌群失调被认为是诱发糖尿病肠道病变的关键因素。 普拉梭菌作为肠道中最为常见的共生菌, 在维持肠道内环境稳态中扮演着重要的角色。前沿研究发现, 源于普拉梭菌的活性产物:微生物抗炎分子(MAM)对炎症性肠病具有炎症抑制潜能。因此, 我们在本研究中探索 MAM 在糖尿病肠道上皮中的功能及机制。 方法: 我们运用 16S 高通量测序分析 db/db 小鼠(2 型糖尿病小鼠模型)的肠道菌群。其次, 通过合成 FLAG 标记的 MAM 质粒, 并进行结肠上皮细胞转染, 以探索蛋白-蛋白互作机制并观测细胞间通透性改变情况。在体内实验中, 我们构建起 E.coli BL21(DE3)细菌蛋白表达系统用于合成 His 标签重组 MAM 蛋白。运用所合成提纯的重组 MAM 蛋白对 db/db 小鼠进行肠道干预, 观察体内干预后肠道上皮屏障结构和功能改善情况。 结果: db/db 小鼠肠道内普拉梭菌丰度显著下降。免疫沉淀联合蛋白质谱分析提示 MAM 可能与细胞紧密连接通路相关蛋白相互作用, 其中一个可能的靶点为 ZO-1。经过 FLAG 标记的MAM 质粒转染后, NCM460、Caco2 及 HT-29 肠道细胞的肠道屏障功能增强, 伴随着 ZO-1表达量的上调。与之相对应的, db/db 小鼠经过 His 标签 MAM 重组蛋白肠道干预后, 显示其肠道屏障功能得到改善, 并且 ZO-1 的表达量也有明显提升。 结论: 我们的研究表明来源于普拉梭菌的 MAM 可通过调控紧密连接通路及ZO-1表达, 修复糖尿病状态下受损的肠道屏障及功能。.
Keywords: Faecalibacterium prausnitzii; diabetes mellitus; diabetic pathology; gut microbiota; intestinal barrier; 普拉梭菌; 糖尿病; 糖尿病病变; 肠道屏障; 肠道菌群.
© 2019 The Authors. Journal of Diabetes published by Ruijin Hospital, Shanghai Jiaotong University School of Medicine and John Wiley & Sons Australia, Ltd.
Figures




Similar articles
-
Barrier Protection and Recovery Effects of Gut Commensal Bacteria on Differentiated Intestinal Epithelial Cells In Vitro.Nutrients. 2020 Jul 28;12(8):2251. doi: 10.3390/nu12082251. Nutrients. 2020. PMID: 32731411 Free PMC article.
-
Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice.Gut Microbes. 2015;6(1):1-9. doi: 10.4161/19490976.2014.990784. Epub 2015 Jan 14. Gut Microbes. 2015. PMID: 25517879 Free PMC article.
-
Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease.Gut. 2016 Mar;65(3):415-425. doi: 10.1136/gutjnl-2014-307649. Epub 2015 Jun 4. Gut. 2016. PMID: 26045134 Free PMC article.
-
Action and function of Faecalibacterium prausnitzii in health and disease.Best Pract Res Clin Gastroenterol. 2017 Dec;31(6):643-648. doi: 10.1016/j.bpg.2017.09.011. Epub 2017 Sep 18. Best Pract Res Clin Gastroenterol. 2017. PMID: 29566907 Review.
-
Epithelial Barrier Function in Gut-Bone Signaling.Adv Exp Med Biol. 2017;1033:151-183. doi: 10.1007/978-3-319-66653-2_8. Adv Exp Med Biol. 2017. PMID: 29101655 Free PMC article. Review.
Cited by
-
Dysbiosis of Gut Microbiota and Short-Chain Fatty Acids in Encephalitis: A Chinese Pilot Study.Front Immunol. 2020 Aug 20;11:1994. doi: 10.3389/fimmu.2020.01994. eCollection 2020. Front Immunol. 2020. PMID: 32973805 Free PMC article.
-
Treatment of Diabetes Nephropathy in Mice by Germinating Seeds of Euryale ferox through Improving Oxidative Stress.Foods. 2023 Feb 9;12(4):767. doi: 10.3390/foods12040767. Foods. 2023. PMID: 36832842 Free PMC article.
-
Pronounced gut microbiota signatures in patients with JAK2V617F-positive essential thrombocythemia.Microbiol Spectr. 2023 Sep 11;11(5):e0066223. doi: 10.1128/spectrum.00662-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37695126 Free PMC article.
-
Effects of Helicobacter pylori treatment on the incidences of autoimmune diseases and inflammatory bowel disease in patients with diabetes mellitus.PLoS One. 2022 May 23;17(5):e0265323. doi: 10.1371/journal.pone.0265323. eCollection 2022. PLoS One. 2022. PMID: 35604898 Free PMC article.
-
GLP-1 receptor agonists modulate blood glucose levels in T2DM by affecting Faecalibacterium prausnitzii abundance in the intestine.Medicine (Baltimore). 2023 Sep 1;102(35):e34978. doi: 10.1097/MD.0000000000034978. Medicine (Baltimore). 2023. PMID: 37657059 Free PMC article.
References
-
- Min XH, Yu T, Qing Q, et al. Abnormal differentiation of intestinal epithelium and intestinal barrier dysfunction in diabetic mice associated with depressed Notch/NICD transduction in Notch/Hes1 signal pathway. Cell Biol Int. 2014;38:1194‐1204. - PubMed
-
- Pasini E, Corsetti G, Assanelli D, et al. Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva Med. 2019;110:3‐11. - PubMed
-
- Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376‐1383. - PubMed
-
- Klüppelholz B, Thorand B, Koenig W, et al. Association of subclinical inflammation with deterioration of glycaemia before the diagnosis of type 2 diabetes: the KORA S4/F4 study. Diabetologia. 2015;58:2269‐2277. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

