GSK-3β inhibition protects the rat heart from the lipopolysaccharide-induced inflammation injury via suppressing FOXO3A activity
- PMID: 31503410
- PMCID: PMC6815822
- DOI: 10.1111/jcmm.14656
GSK-3β inhibition protects the rat heart from the lipopolysaccharide-induced inflammation injury via suppressing FOXO3A activity
Abstract
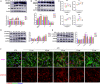
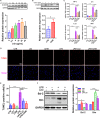
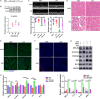
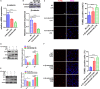
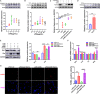
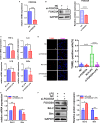
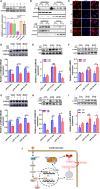
Sepsis-induced cardiac dysfunction represents a main cause of death in intensive care units. Previous studies have indicated that GSK-3β is involved in the modulation of sepsis. However, the signalling details of GSK-3β regulation in endotoxin lipopolysaccharide (LPS)-induced septic myocardial dysfunction are still unclear. Here, based on the rat septic myocardial injury model, we found that LPS could induce GSK-3β phosphorylation at its active site (Y216) and up-regulate FOXO3A level in primary cardiomyocytes. The FOXO3A expression was significantly reduced by GSK-3β inhibitors and further reversed through β-catenin knock-down. This pharmacological inhibition of GSK-3β attenuated the LPS-induced cell injury via mediating β-catenin signalling, which could be abolished by FOXO3A activation. In vivo, GSK-3β suppression consistently improved cardiac function and relieved heart injury induced by LPS. In addition, the increase in inflammatory cytokines in LPS-induced model was also blocked by inhibition of GSK-3β, which curbed both ERK and NF-κB pathways, and suppressed cardiomyocyte apoptosis via activating the AMP-activated protein kinase (AMPK). Our results demonstrate that GSK-3β inhibition attenuates myocardial injury induced by endotoxin that mediates the activation of FOXO3A, which suggests a potential target for the therapy of septic cardiac dysfunction.
Keywords: FOXO3A; GSK-3β; NF-κB; cardiac dysfunction; inflammation injury; sepsis.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there is no conflict of interest.
Figures
Similar articles
-
Kcnh2 mediates FAK/AKT-FOXO3A pathway to attenuate sepsis-induced cardiac dysfunction.Cell Prolif. 2021 Feb;54(2):e12962. doi: 10.1111/cpr.12962. Epub 2020 Dec 2. Cell Prolif. 2021. PMID: 33263944 Free PMC article.
-
Apoptosis of cardiomyocytes in diabetic cardiomyopathy involves overexpression of glycogen synthase kinase-3β.Biosci Rep. 2019 Jan 3;39(1):BSR20171307. doi: 10.1042/BSR20171307. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30237226 Free PMC article.
-
Glycogen synthase kinase-3 beta inhibitor suppresses Porphyromonas gingivalis lipopolysaccharide-induced CD40 expression by inhibiting nuclear factor-kappa B activation in mouse osteoblasts.Mol Immunol. 2012 Aug;52(1):38-49. doi: 10.1016/j.molimm.2012.04.005. Epub 2012 May 14. Mol Immunol. 2012. PMID: 22580404
-
The signaling interplay of GSK-3β in myocardial disorders.Drug Discov Today. 2020 Apr;25(4):633-641. doi: 10.1016/j.drudis.2020.01.017. Epub 2020 Jan 31. Drug Discov Today. 2020. PMID: 32014454 Review.
-
The role of glycogen synthase kinase 3 beta in multiple sclerosis.Biomed Pharmacother. 2020 Dec;132:110874. doi: 10.1016/j.biopha.2020.110874. Epub 2020 Oct 18. Biomed Pharmacother. 2020. PMID: 33080467 Review.
Cited by
-
Pterostilbene attenuates heart failure by inhibiting myocardial ferroptosis through SIRT1/GSK-3β/GPX4 signaling pathway.Heliyon. 2024 Jan 20;10(3):e24562. doi: 10.1016/j.heliyon.2024.e24562. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38318046 Free PMC article.
-
Sufentanil Alleviates Sepsis-Induced Myocardial Injury and Stress Response in Rats through the ERK/GSK-3β Signaling Axis.Evid Based Complement Alternat Med. 2022 Jun 21;2022:9630716. doi: 10.1155/2022/9630716. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Aug 2;2023:9854369. doi: 10.1155/2023/9854369. PMID: 35774755 Free PMC article. Retracted.
-
STAT3/FoxO3a/Sirt1 pathway inhibition by ginsenoside Rc ameliorates cardiomyocyte damage in septic cardiomyopathy by altering macrophage polarization.J Mol Histol. 2025 Apr 28;56(3):148. doi: 10.1007/s10735-025-10417-3. J Mol Histol. 2025. PMID: 40293549
-
miR-29b-1-5p exacerbates myocardial injury induced by sepsis in a mouse model by targeting TERF2.Acta Biochim Biophys Sin (Shanghai). 2024 Apr 25;56(4):607-620. doi: 10.3724/abbs.2024020. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38414350 Free PMC article.
-
Beneficial role of oleuropein in sepsis-induced myocardial injury. Possible Involvement of GSK-3β/NF-kB pathway.Acta Cir Bras. 2021 Feb 15;36(1):e360107. doi: 10.1590/ACB360107. eCollection 2021. Acta Cir Bras. 2021. PMID: 33605309 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

