Exploring the bi-directional relationship between autophagy and Alzheimer's disease
- PMID: 31503421
- PMCID: PMC6978262
- DOI: 10.1111/cns.13216
Exploring the bi-directional relationship between autophagy and Alzheimer's disease
Abstract
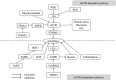
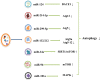
Alzheimer's disease (AD) is characterized by β-amyloid (Aβ) deposition and Tau phosphorylation, in which its pathogenesis has not been cleared so far. The metabolism of Aβ and Tau is critically affected by the autophagy. Abnormal autophagy is thought to be involved in the pathogenesis of AD, regulating autophagy may become a new strategy for AD treatment. In the early stage of AD, the presence of Aβ and Tau can induce autophagy to promote their clearance by means of mTOR-dependent and independent manners. As AD progress, the autophagy goes aberrant. As a result, Aβ and Tau generate continually, which aggravates both autophagy dysfunction and AD. Besides, several related genes and proteins of AD can also adapt autophagy to make an effect on the AD development. There seems to be a bi-directional relationship between AD pathology and autophagy. At present, this article reviews this relationship from these aspects: (a) the signaling pathways of regulating autophagy; (b) the relationships between the autophagy and the processing of Aβ; (c) Aβ and Tau cause autophagy dysfunction; (d) normal autophagy promotes the clearance of Aβ and Tau; (e) the relationships between the autophagy and both genes and proteins related to AD: TFEB, miRNAs, Beclin-1, Presenilin, and Nrf2; and (f) the small molecules regulating autophagy on AD therapy. All of the above may help to further elucidate the pathogenesis of AD and provide a theoretical basis for clinical treatment of AD.
Keywords: Alzheimer's disease; Tau; autophagy; genes and proteins; β-amyloid.
© 2019 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Wu M‐Y, Song J‐X, Wang S‐F, Cai C‐Z, Li M, Lu J‐H. Selective autophagy: the new player in the fight against neurodegenerative diseases? Brain Res Bull. 2018;137:79‐90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

