A rare case of acquired immunodeficiency associated with myelodysplastic syndrome
- PMID: 31503426
- PMCID: PMC6825869
- DOI: 10.1002/mgg3.923
A rare case of acquired immunodeficiency associated with myelodysplastic syndrome
Abstract
Background: Pediatric myelodysplastic syndromes (MDS) display clonal genomic instability that can lead to acquisition of other hematological disorders, usually by loss of heterozygosity. Immunodeficiency caused by uniparental disomy (UPD) has not previously been reported.
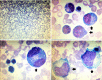
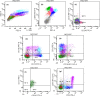
Methods: We investigated a 13-year-old boy who suffered from recurrent infections and pancytopenia for 1 year. Both the comet assay and chromosome breakage analysis were normal, but the bone marrow showed evidence of dysplasia characteristic of MDS. With his normal sister as donor, he underwent failed hematopoietic stem cell transplantation (HSCT) with reduced intensity conditioning (RIC) followed by successful HSCT with myeloablative conditioning (MAC). We used single nucleotide polymorphism (SNP) array, targeted gene panel, and whole exome sequencing to investigate the etiology of his disease.
Results: The molecular analyses revealed multiple regions of homozygosity, one region encompassing a homozygous missense variant of recombination activating gene 1 (RAG1) which was previously associated with severe immunodeficiency in infancy. This RAG1 mutation was heterozygous in the proband's fingernail DNA, but was changed to homozygous in the proband's marrow by somatic acquisition of UPD event. No other pathogenic driver mutation for MDS-related genes was identified.
Conclusion: The hematological phenotype, somatic genomic instability, and response to HSCT MAC but not HSCT RIC deduced to a diagnosis of MDS type refractory cytopenia of children in this patient. His immunodeficiency was secondary to MDS due to somatic acquisition of homozygosity for known pathogenic RAG1 mutation.
Keywords: RAG1; acquired UPD; immunodeficiency; myelodysplastic syndrome.
© 2019 Capital Institute of Pediatrics. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Fitzgibbon, J. , Smith, L.‐L. , Raghavan, M. , Smith, M. L. , Debernardi, S. , Skoulakis, S. , … Young, B. D. (2005). Association between acquired uniparental disomy and homozygous gene mutation in acute myeloid leukemias. Cancer Research, 65(20), 9152–9154. 10.1158/0008-5472.CAN-05-2017 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

