Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors
- PMID: 31503443
- PMCID: PMC6837877
- DOI: 10.1021/acsnano.9b02395
Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors
Abstract
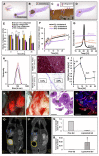
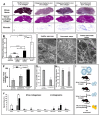
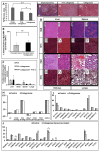
Overexpressed extracellular matrix (ECM) in pancreatic ductal adenocarcinoma (PDAC) limits drug penetration into the tumor and is associated with poor prognosis. Here, we demonstrate that a pretreatment based on a proteolytic-enzyme nanoparticle system disassembles the dense PDAC collagen stroma and increases drug penetration into the pancreatic tumor. More specifically, the collagozome, a 100 nm liposome encapsulating collagenase, was rationally designed to protect the collagenase from premature deactivation and prolonged its release rate at the target site. Collagen is the main component of the PDAC stroma, reaching 12.8 ± 2.3% vol in diseased mice pancreases, compared to 1.4 ± 0.4% in healthy mice. Upon intravenous injection of the collagozome, ∼1% of the injected dose reached the pancreas over 8 h, reducing the level of fibrotic tissue to 5.6 ± 0.8%. The collagozome pretreatment allowed increased drug penetration into the pancreas and improved PDAC treatment. PDAC tumors, pretreated with the collagozome followed by paclitaxel micelles, were 87% smaller than tumors pretreated with empty liposomes followed by paclitaxel micelles. Interestingly, degrading the ECM did not increase the number of circulating tumor cells or metastasis. This strategy holds promise for degrading the extracellular stroma in other diseases as well, such as liver fibrosis, enhancing tissue permeability before drug administration.
Keywords: collagen; extracellular matrix; fibrosis; liposome; nanoparticle; paclitaxel micelles; pancreatic cancer.
Figures

Similar articles
-
Co-encapsulation of collagenase type I and silibinin in chondroitin sulfate coated multilayered nanoparticles for targeted treatment of liver fibrosis.Carbohydr Polym. 2021 Jul 1;263:117964. doi: 10.1016/j.carbpol.2021.117964. Epub 2021 Mar 24. Carbohydr Polym. 2021. PMID: 33858569
-
A collagenase-decorated Cu-based nanotheranostics: remodeling extracellular matrix for optimizing cuproptosis and MRI in pancreatic ductal adenocarcinoma.J Nanobiotechnology. 2024 Nov 10;22(1):689. doi: 10.1186/s12951-024-02968-6. J Nanobiotechnology. 2024. PMID: 39523309 Free PMC article.
-
Partial ligand shielding nanoparticles improve pancreatic ductal adenocarcinoma treatment via a multifunctional paradigm for tumor stroma reprogramming.Acta Biomater. 2022 Jun;145:122-134. doi: 10.1016/j.actbio.2022.03.050. Epub 2022 Apr 2. Acta Biomater. 2022. PMID: 35381402
-
Extensive Review of Nanomedicine Strategies Targeting the Tumor Microenvironment in PDAC.Int J Nanomedicine. 2025 Mar 17;20:3379-3406. doi: 10.2147/IJN.S504503. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40125427 Free PMC article. Review.
-
Digesting a Path Forward: The Utility of Collagenase Tumor Treatment for Improved Drug Delivery.Mol Pharm. 2018 Jun 4;15(6):2069-2083. doi: 10.1021/acs.molpharmaceut.8b00319. Epub 2018 May 16. Mol Pharm. 2018. PMID: 29767984 Free PMC article. Review.
Cited by
-
Design of Nanoparticles in Cancer Therapy Based on Tumor Microenvironment Properties.Pharmaceutics. 2022 Dec 3;14(12):2708. doi: 10.3390/pharmaceutics14122708. Pharmaceutics. 2022. PMID: 36559202 Free PMC article. Review.
-
Overcoming Physiological Barriers to Nanoparticle Delivery-Are We There Yet?Front Bioeng Biotechnol. 2019 Dec 17;7:415. doi: 10.3389/fbioe.2019.00415. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31921819 Free PMC article. Review.
-
A Novel Bionic Catalyst-Mediated Drug Delivery System for Enhanced Sonodynamic Therapy.Front Bioeng Biotechnol. 2021 Jul 30;9:699737. doi: 10.3389/fbioe.2021.699737. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34395406 Free PMC article.
-
Sequential drug release system: Targeting the tumor ECM for enhanced chemotherapy efficacy.Proc Natl Acad Sci U S A. 2025 Jun 10;122(23):e2421061122. doi: 10.1073/pnas.2421061122. Epub 2025 Jun 5. Proc Natl Acad Sci U S A. 2025. PMID: 40472035
-
Epigenetic regulation and targeting of ECM for cancer therapy.Am J Physiol Cell Physiol. 2022 Apr 1;322(4):C762-C768. doi: 10.1152/ajpcell.00022.2022. Epub 2022 Mar 2. Am J Physiol Cell Physiol. 2022. PMID: 35235427 Free PMC article. Review.
References
-
- Neesse A, Algul H, Tuveson DA, Gress TM. Stromal Biology and Therapy in Pancreatic Cancer: A Changing Paradigm. Gut. 2015;64:1476–1484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

