Proteolytic Control of Lipid Metabolism
- PMID: 31503446
- PMCID: PMC6989095
- DOI: 10.1021/acschembio.9b00695
Proteolytic Control of Lipid Metabolism
Abstract
Synthesis and regulation of lipid levels and identities is critical for a wide variety of cellular functions, including structural and morphological properties of organelles, energy storage, signaling, and stability and function of membrane proteins. Proteolytic cleavage events regulate and/or influence some of these lipid metabolic processes and as a result help modulate their pleiotropic cellular functions. Proteins involved in lipid regulation are proteolytically cleaved for the purpose of their relocalization, processing, turnover, and quality control, among others. The scope of this review includes proteolytic events governing cellular lipid dynamics. After an initial discussion of the classic example of sterol regulatory element-binding proteins, our focus will shift to the mitochondrion, where a range of proteolytic events are critical for normal mitochondrial phospholipid metabolism and enforcing quality control therein. Recently, mitochondrial phospholipid metabolic pathways have been implicated as important for the proliferative capacity of cancers. Thus, the assorted proteases that regulate, monitor, or influence the activity of proteins that are important for phospholipid metabolism represent attractive targets to be manipulated for research purposes and clinical applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



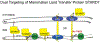
Similar articles
-
Mitochondrial Proteases: Multifaceted Regulators of Mitochondrial Plasticity.Annu Rev Biochem. 2020 Jun 20;89:501-528. doi: 10.1146/annurev-biochem-062917-012739. Epub 2020 Feb 19. Annu Rev Biochem. 2020. PMID: 32075415 Review.
-
Mitochondrial proteolysis: its emerging roles in stress responses.Biochim Biophys Acta. 2015 Feb;1850(2):274-80. doi: 10.1016/j.bbagen.2014.10.012. Epub 2014 Oct 23. Biochim Biophys Acta. 2015. PMID: 25459516 Review.
-
The role of AAA+ proteases in mitochondrial protein biogenesis, homeostasis and activity control.Subcell Biochem. 2013;66:223-63. doi: 10.1007/978-94-007-5940-4_9. Subcell Biochem. 2013. PMID: 23479443 Review.
-
Control of Adhesion GPCR Function Through Proteolytic Processing.Handb Exp Pharmacol. 2016;234:83-109. doi: 10.1007/978-3-319-41523-9_5. Handb Exp Pharmacol. 2016. PMID: 27832485 Review.
-
Mitochondrial proteases act on STARD3 to activate progesterone synthesis in human syncytiotrophoblast.Biochim Biophys Acta. 2015 Jan;1850(1):107-17. doi: 10.1016/j.bbagen.2014.10.009. Epub 2014 Oct 18. Biochim Biophys Acta. 2015. PMID: 25459514
Cited by
-
Free-Radical-Mediated Formation of Trans-Cardiolipin Isomers, Analytical Approaches for Lipidomics and Consequences of the Structural Organization of Membranes.Biomolecules. 2020 Aug 15;10(8):1189. doi: 10.3390/biom10081189. Biomolecules. 2020. PMID: 32824246 Free PMC article.
-
Impaired phosphatidylethanolamine metabolism activates a reversible stress response that detects and resolves mutant mitochondrial precursors.iScience. 2021 Feb 16;24(3):102196. doi: 10.1016/j.isci.2021.102196. eCollection 2021 Mar 19. iScience. 2021. PMID: 33718843 Free PMC article.
-
Role of Yme1 in mitochondrial protein homeostasis: from regulation of protein import, OXPHOS function to lipid synthesis and mitochondrial dynamics.Biochem Soc Trans. 2024 Jun 26;52(3):1539-1548. doi: 10.1042/BST20240450. Biochem Soc Trans. 2024. PMID: 38864432 Free PMC article. Review.
-
Ubiquitinated AIF is a major mediator of hypoxia-induced mitochondrial dysfunction and pulmonary artery smooth muscle cell proliferation.Cell Biosci. 2022 Jan 28;12(1):9. doi: 10.1186/s13578-022-00744-3. Cell Biosci. 2022. PMID: 35090552 Free PMC article.
-
Lipid Metabolism and Cancer.Life (Basel). 2022 May 25;12(6):784. doi: 10.3390/life12060784. Life (Basel). 2022. PMID: 35743814 Free PMC article. Review.
References
-
- Singer SJ, and Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175, 720–731. - PubMed
-
- Ohvo-Rekilä H, Ramstedt B, Leppimäki P, and Slotte JP. (2002) Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res 41, 66–97. - PubMed
-
- Bretscher MS (1972) Asymmetrical lipid bilayer structure for biological membranes. Nat. New Biol 236, 11–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

