Sequence-Defined Macrocycles for Understanding and Controlling the Build-up of Hierarchical Order in Self-Assembled 2D Arrays
- PMID: 31503483
- PMCID: PMC7461245
- DOI: 10.1021/jacs.9b06410
Sequence-Defined Macrocycles for Understanding and Controlling the Build-up of Hierarchical Order in Self-Assembled 2D Arrays
Abstract
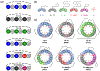
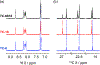
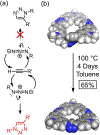
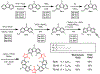
Anfinsen's dogma that sequence dictates structure is fundamental to understanding the activity and assembly of proteins. This idea has been applied to all manner of oligomers but not to the behavior of cyclic oligomers, aka macrocycles. We do this here by providing the first proofs that sequence controls the hierarchical assembly of nonbiological macrocycles, in this case, at graphite surfaces. To design macrocycles with one (AAA), two (AAB), or three (ABC) different carbazole units, we needed to subvert the synthetic preferences for one-pot macrocyclizations. We developed a new stepwise synthesis with sequence-defined targets made in 11, 17, and 22 steps with 25, 10, and 5% yields, respectively. The linear build up of primary sequence (1°) also enabled a thermal Huisgen cycloaddition to proceed regioselectively for the first time using geometric control. The resulting macrocycles are planar (2° structure) and form H-bonded dimers (3°) at surfaces. Primary sequences encoded into the suite of tricarb macrocycles were shown by scanning-tunneling microscopy (STM) to impact the next levels of supramolecular ordering (4°) and 2D crystalline polymorphs (5°) at solution-graphite interfaces. STM imaging of an AAB macrocycle revealed the formation of a new gap phase that was inaccessible using only C3-symmetric macrocycles. STM imaging of two additional sequence-controlled macrocycles (AAD, ABE) allowed us to identify the factors driving the formation of this new polymorph. This demonstration of how sequence controls the hierarchical patterning of macrocycles raises the importance of stepwise syntheses relative to one-pot macrocyclizations to offer new approaches for greater understanding and control of hierarchical assembly.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


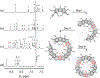
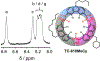






Similar articles
-
Assembly of macrocycles by zirconocene-mediated, reversible carbon-carbon bond formation.Acc Chem Res. 2011 Jun 21;44(6):435-46. doi: 10.1021/ar100148g. Epub 2011 Apr 7. Acc Chem Res. 2011. PMID: 21473633
-
Multifunctional Tricarbazolo Triazolophane Macrocycles: One-Pot Preparation, Anion Binding, and Hierarchical Self-Organization of Multilayers.Chemistry. 2016 Jan 11;22(2):560-9. doi: 10.1002/chem.201503161. Epub 2015 Nov 23. Chemistry. 2016. PMID: 26593327
-
Breaking Radial Dipole Symmetry in Planar Macrocycles Modulates Edge-to-Edge Packing and Disrupts Cofacial Stacking.Chemistry. 2024 Feb 7;30(8):e202302946. doi: 10.1002/chem.202302946. Epub 2023 Dec 19. Chemistry. 2024. PMID: 37950681
-
Hydrogen-bonded aromatic amide macrocycles: synthesis, properties and functions.Org Biomol Chem. 2022 Nov 30;20(46):9023-9051. doi: 10.1039/d2ob01263d. Org Biomol Chem. 2022. PMID: 36128982 Review.
-
Creating molecular macrocycles for anion recognition.Beilstein J Org Chem. 2016 Mar 31;12:611-27. doi: 10.3762/bjoc.12.60. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27340452 Free PMC article. Review.
Cited by
-
Chiral, sequence-definable foldamer-derived macrocycles.Chem Sci. 2021 Nov 10;12(47):15632-15636. doi: 10.1039/d1sc05021d. eCollection 2021 Dec 8. Chem Sci. 2021. PMID: 35003593 Free PMC article.
-
Iterative Exponential Growth of Oxygen-Linked Aromatic Polymers Driven by Nucleophilic Aromatic Substitution Reactions.Front Chem. 2021 Apr 28;9:620017. doi: 10.3389/fchem.2021.620017. eCollection 2021. Front Chem. 2021. PMID: 33996739 Free PMC article.
-
Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds.Chem Rev. 2022 Jan 12;122(1):565-788. doi: 10.1021/acs.chemrev.1c00449. Epub 2021 Dec 1. Chem Rev. 2022. PMID: 34850633 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

