Paracrine crosstalk between intestinal L- and D-cells controls secretion of glucagon-like peptide-1 in mice
- PMID: 31503512
- PMCID: PMC6962500
- DOI: 10.1152/ajpendo.00239.2019
Paracrine crosstalk between intestinal L- and D-cells controls secretion of glucagon-like peptide-1 in mice
Abstract
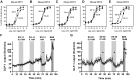
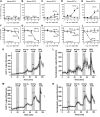
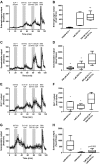
DPP-4 inhibitors, used for treatment of type 2 diabetes, act by increasing the concentrations of intact glucagon-like peptide-1 (GLP-1), but at the same time, they inhibit secretion of GLP-1, perhaps by a negative feedback mechanism. We hypothesized that GLP-1 secretion is feedback regulated by somatostatin (SS) from neighboring D-cells, and blocking this feedback circuit results in increased GLP-1 secretion. We used a wide range of experimental techniques, including gene expression analysis, immunohistochemical approaches, and the perfused mouse intestine to characterize the paracrine circuit controlling GLP-1 and SS. We show that 1) antagonizing the SS receptor (SSTr) 2 and SSTr5 led to increased GLP-1 and SS secretion in the mouse, 2) SS exhibits strong tonic inhibition of GLP-1 secretion preferentially through SSTr5, and 3) the secretion of S was GLP-1 receptor dependent. We conclude that SS is a tonic inhibitor of GLP-1 secretion, and interventions in the somatostain-GLP-1 paracrine loop lead to increased GLP-1 secretion.
Keywords: GLP-1; SSTr antagonist; paracrine loop; somatostatin; somatostatin receptors.
Conflict of interest statement
A. D. Christ and R. E. Martin (F. Hoffmann-La Roche Ltd.) hold a patent on the SSTr5 antagonist (Compound B), patent no. WO 2008019967A3.
Figures



Similar articles
-
Antagonizing somatostatin receptor subtype 2 and 5 reduces blood glucose in a gut- and GLP-1R-dependent manner.JCI Insight. 2021 Feb 22;6(4):e143228. doi: 10.1172/jci.insight.143228. JCI Insight. 2021. PMID: 33434183 Free PMC article.
-
Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures.Am J Physiol Endocrinol Metab. 2002 Aug;283(2):E311-7. doi: 10.1152/ajpendo.00434.2001. Am J Physiol Endocrinol Metab. 2002. PMID: 12110536
-
Truncated and full-length glucagon-like peptide-1 (GLP-1) differentially stimulate intestinal somatostatin release.Endocrine. 1997 Feb;6(1):91-5. doi: 10.1007/BF02738808. Endocrine. 1997. PMID: 9225122
-
Novel extrapancreatic effects of incretin.J Diabetes Investig. 2016 Apr;7 Suppl 1(Suppl 1):76-9. doi: 10.1111/jdi.12495. Epub 2016 Mar 31. J Diabetes Investig. 2016. PMID: 27186360 Free PMC article. Review.
-
Intestinal regulation of urinary sodium excretion and the pathophysiology of diabetic kidney disease: a focus on glucagon-like peptide 1 and dipeptidyl peptidase 4.Exp Physiol. 2014 Sep;99(9):1140-5. doi: 10.1113/expphysiol.2014.078766. Epub 2014 Aug 1. Exp Physiol. 2014. PMID: 25085841 Free PMC article. Review.
Cited by
-
Versatile Functions of Somatostatin and Somatostatin Receptors in the Gastrointestinal System.Front Endocrinol (Lausanne). 2021 Mar 16;12:652363. doi: 10.3389/fendo.2021.652363. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33796080 Free PMC article. Review.
-
Predicting cell-to-cell communication networks using NATMI.Nat Commun. 2020 Oct 6;11(1):5011. doi: 10.1038/s41467-020-18873-z. Nat Commun. 2020. PMID: 33024107 Free PMC article.
-
Glucose- and Bile Acid-Stimulated Secretion of Gut Hormones in the Isolated Perfused Intestine Is Not Impaired in Diet-Induced Obese Mice.Front Endocrinol (Lausanne). 2022 May 4;13:884501. doi: 10.3389/fendo.2022.884501. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35600607 Free PMC article.
-
Antagonizing somatostatin receptor subtype 2 and 5 reduces blood glucose in a gut- and GLP-1R-dependent manner.JCI Insight. 2021 Feb 22;6(4):e143228. doi: 10.1172/jci.insight.143228. JCI Insight. 2021. PMID: 33434183 Free PMC article.
-
GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management.Nat Metab. 2024 Oct;6(10):1866-1885. doi: 10.1038/s42255-024-01113-9. Epub 2024 Aug 19. Nat Metab. 2024. PMID: 39160334 Review.
References
-
- Bak MJ, Wewer Albrechtsen NJ, Pedersen J, Knop FK, Vilsbøll T, Jørgensen NB, Hartmann B, Deacon CF, Dragsted LO, Holst JJ. Specificity and sensitivity of commercially available assays for glucagon-like peptide-1 (GLP-1): implications for GLP-1 measurements in clinical studies. Diabetes Obes Metab 16: 1155–1164, 2014. doi:10.1111/dom.12352. - DOI - PubMed
-
- Baldissera FG, Holst JJ, Knuhtsen S, Hilsted L, Nielsen OV. Oxyntomodulin (glicentin-(33-69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul Pept 21: 151–166, 1988. doi:10.1016/0167-0115(88)90099-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

