Novel application of amino-acid buffered solution for neuroprotection against ischemia/reperfusion injury
- PMID: 31504040
- PMCID: PMC6736298
- DOI: 10.1371/journal.pone.0221039
Novel application of amino-acid buffered solution for neuroprotection against ischemia/reperfusion injury
Abstract
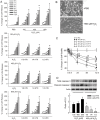
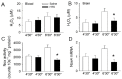
Ischemic neuron loss contributes to brain dysfunction in patients with cardiac arrest (CA). Histidine-tryptophan-ketoglutarate (HTK) solution is a preservative used during organ transplantation. We tested the potential of HTK to protect neurons from severe hypoxia (SH) following CA. We isolated rat primary cortical neurons and induced SH with or without HTK. Changes in caspase-3, hypoxia-inducible factor 1-alpha (HIF-1α), and nicotinamide adenine dinucleotide phosphate oxidase-4 (NOX4) expression were evaluated at different time points up to 72 h. Using a rat asphyxia model, we induced CA-mediated brain damage and then completed resuscitation. HTK or sterile saline was administered into the left carotid artery. Neurological deficit scoring and mortality were evaluated for 3 days. Then the rats were sacrificed for evaluation of NOX4 and H2O2 levels in blood and brain. In the in vitro study, HTK attenuated SH- and H2O2-mediated cytotoxicity in a volume- and time-dependent manner, associated with persistent HIF-1α expression and reductions in procaspase-3 activation and NOX4 expression. The inhibition of HIF-1α abrogated HTK's effect on NOX4. In the in vivo study, neurological scores were significantly improved by HTK. H2O2 level, NOX4 activity, and NOX4 gene expression were all decreased in the brain specimens of HTK-treated rats. Our results suggest that HTK acts as an effective neuroprotective solution by maintaining elevated HIF-1α level, which was associated with inhibited procaspase-3 activation and decreased NOX4 expression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway.Chem Biol Interact. 2018 Mar 25;284:32-40. doi: 10.1016/j.cbi.2018.02.017. Epub 2018 Feb 16. Chem Biol Interact. 2018. PMID: 29454613
-
Salviaolate Protects Rat Brain from Ischemia-Reperfusion Injury through Inhibition of NADPH Oxidase.Planta Med. 2015 Oct;81(15):1361-9. doi: 10.1055/s-0035-1557774. Epub 2015 Aug 7. Planta Med. 2015. PMID: 26252829
-
Vitexin reduces hypoxia-ischemia neonatal brain injury by the inhibition of HIF-1alpha in a rat pup model.Neuropharmacology. 2015 Dec;99:38-50. doi: 10.1016/j.neuropharm.2015.07.007. Epub 2015 Jul 14. Neuropharmacology. 2015. PMID: 26187393
-
Custodiol-N™ cardioplegia lowers cerebral inflammation and activation of hypoxia-inducible factor-1α.Interact Cardiovasc Thorac Surg. 2019 Jun 1;28(6):884-892. doi: 10.1093/icvts/ivy347. Interact Cardiovasc Thorac Surg. 2019. PMID: 30668864
-
Partridgeberry polyphenols protect rat primary cortical neurons from oxygen-glucose deprivation-reperfusion-induced injury via suppression of inflammatory adipokines and regulation of HIF-1α and PPARγ.Nutr Neurosci. 2016 Jul;19(6):260-8. doi: 10.1179/1476830515Y.0000000026. Epub 2015 May 5. Nutr Neurosci. 2016. PMID: 25941748
Cited by
-
TRPV1 Hyperfunction Involved in Uremic Toxin Indoxyl Sulfate-Mediated Renal Tubular Damage.Int J Mol Sci. 2020 Aug 27;21(17):6212. doi: 10.3390/ijms21176212. Int J Mol Sci. 2020. PMID: 32867359 Free PMC article.
-
Pharmacological Inhibition of NOX4 Improves Mitochondrial Function and Survival in Human Beta-Cells.Biomedicines. 2021 Dec 8;9(12):1865. doi: 10.3390/biomedicines9121865. Biomedicines. 2021. PMID: 34944680 Free PMC article.
-
TRPV1 Hyperfunction Contributes to Renal Inflammation in Oxalate Nephropathy.Int J Mol Sci. 2021 Jun 8;22(12):6204. doi: 10.3390/ijms22126204. Int J Mol Sci. 2021. PMID: 34201387 Free PMC article.
-
The Role of Dectin-1-Mediated M1 Macrophage Polarization in Cerebral Ischemia-Reperfusion Injury.Emerg Med Int. 2021 May 21;2021:6697271. doi: 10.1155/2021/6697271. eCollection 2021. Emerg Med Int. 2021. PMID: 34094602 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

