Dolutegravir based antiretroviral therapy compared to other combined antiretroviral regimens for the treatment of HIV-infected naive patients: A systematic review and meta-analysis
- PMID: 31504060
- PMCID: PMC6736283
- DOI: 10.1371/journal.pone.0222229
Dolutegravir based antiretroviral therapy compared to other combined antiretroviral regimens for the treatment of HIV-infected naive patients: A systematic review and meta-analysis
Abstract
Background: Numerous randomized clinical trials (RCTs) were conducted to evaluate dolutegravir based triple antiretroviral therapy (ART) compared to other triple antiretroviral regimens in naïve patients, and a summary of the available evidence is required to shed more light on safety and effectiveness issues.
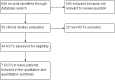
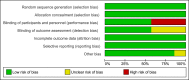
Methods: Systematic review and meta-analysis of RCTs comparing dolutegravir-containing ART to non-dolutegravir containing ART in HIV-infected naive patients. Primary outcomes: % of patients with viral load<50 copies/mL at 48 weeks, stratified according to baseline viral load levels (< or >100.000 copies/mL); overall rate of discontinuation and/or switching for any cause (virologic failure, clinical failure, adverse events). Measure of treatment effect: Risk Difference (RD) with 95% confidence intervals (CIs). The GRADE system was used to assess the certainty of the body of evidence.
Results: We included 7 RCTs (13 reports, 6407patients) comparing dolutegravir containing to non-dolutegravir containing ART, both in combination with 2 NRTIs. Controls were raltegravir or bictegravir (3 RCTs), boosted atazanavir or darunavir (2 RCTs) or efavirenz (2 RCTs). Rates of patients with VL <50 copies/ml were higher in dolutegravir recipients compared to controls at 48 weeks (RD, 0.05; 95% CIs, 0.03/0.08, p = 0.0002) and 96 weeks (RD, 0.06; 95% CIs, 0.03/0.10, p<0.0001); the average benefit of using dolutegravir was particularly evident at 48 weeks in the subgroup of patients with high baseline viral load (RD, 0.10; 95% CIs, 0.05/0.15; p< 0.0001; GRADE assessment: "high certainty of evidence"). Overall rate of discontinuation were lower in dolutegravir compared to controls (RD,-0.03, 95% CIs -0.05/-0.01; p = 0.007). No significant differences were observed in rates of discontinuation due to adverse events (RD, -0.02; 95% CIs, -0.05/0.00), virologic failure (RD, -0.01; 95% CIs, -0.02/0.01), and most common adverse events (GRADE assessment: from "very-low" to "moderate certainty of evidence").
Conclusion: Starting treatment in naive patients with dolutegravir containing ART has an increased likelihood of achieving viral suppression in the comparison with non-dolutegravir containing ART. The average benefit is particularly evident in those with high baseline viral load.
Conflict of interest statement
MC has received honoraria as a speaker and/or advisor from Abbott, Bayer, Cephalon, Gilead, Novartis and ViiV Healthcare. SGP has received research grants from Gilead Sciences, ViiV Healthcare, Abbott, MS&D, and honoraria as a speaker from MS&D, Gilead Sciences, ViiV Healthcare, Abbvie, Janssen. These activities were not related to their work with this review. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study.Lancet. 2013 Mar 2;381(9868):735-43. doi: 10.1016/S0140-6736(12)61853-4. Epub 2013 Jan 8. Lancet. 2013. PMID: 23306000 Clinical Trial.
-
Short Communication: Dolutegravir-Based Regimens Are Active in Integrase Strand Transfer Inhibitor-Naive Patients with Nucleoside Reverse Transcriptase Inhibitor Resistance.AIDS Res Hum Retroviruses. 2018 Apr;34(4):343-346. doi: 10.1089/AID.2017.0184. Epub 2018 Mar 22. AIDS Res Hum Retroviruses. 2018. PMID: 29444582 Free PMC article.
-
Comparative effectiveness of bictegravir versus dolutegravir, raltegravir, and efavirenz-based antiretroviral therapy among treatment-naïve individuals with HIV.Eur J Intern Med. 2025 Mar;133:86-92. doi: 10.1016/j.ejim.2025.01.013. Epub 2025 Jan 21. Eur J Intern Med. 2025. PMID: 39843330
-
Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection.Ann Pharmacother. 2014 Mar;48(3):395-403. doi: 10.1177/1060028013513558. Epub 2013 Nov 19. Ann Pharmacother. 2014. PMID: 24259658 Review.
-
[Position of dolutegravir in the treatment of HIV infection].Enferm Infecc Microbiol Clin. 2015 Mar;33 Suppl 1:31-3. doi: 10.1016/S0213-005X(15)30007-0. Enferm Infecc Microbiol Clin. 2015. PMID: 25858610 Review. Spanish.
Cited by
-
"No One Needs to be Forced": Qualitative Insights on Competing Priorities between Antiretroviral Therapy and Reproductive Health Planning during the Dolutegravir Rollout.AIDS Behav. 2024 Nov;28(11):3719-3732. doi: 10.1007/s10461-024-04454-4. Epub 2024 Jul 31. AIDS Behav. 2024. PMID: 39083152
-
Efficacy and safety of ainuovirine versus efavirenz combination therapies with lamivudine/tenofovir disoproxil fumarate for medication of treatment-naïve HIV-1-positive adults: week 48 results of a randomized controlled phase 3 clinical trial followed by an open-label setting until week 96.Lancet Reg Health West Pac. 2023 Apr 24;36:100769. doi: 10.1016/j.lanwpc.2023.100769. eCollection 2023 Jul. Lancet Reg Health West Pac. 2023. PMID: 37547039 Free PMC article.
-
Effectiveness and safety of dolutegravir two-drug regimens in virologically suppressed people living with HIV: a systematic literature review and meta-analysis of real-world evidence.HIV Med. 2021 Jul;22(6):423-433. doi: 10.1111/hiv.13050. Epub 2021 Feb 2. HIV Med. 2021. PMID: 33529489 Free PMC article.
-
Change in Blood Pressure, Weight, and Other Cardiovascular Disease Risk Factors After Switch From Protease Inhibitors to Dolutegravir: Post hoc Analysis of the 48-week Randomised Second-line Switch to Dolutegravir Trial.Clin Infect Dis. 2025 Apr 30;80(4):889-892. doi: 10.1093/cid/ciae514. Clin Infect Dis. 2025. PMID: 39589132 Free PMC article. Clinical Trial.
-
Virological Suppression and Its Associated Factors of Dolutegravir Based Regimen in a Resource-Limited Setting: An Observational Retrospective Study in Ethiopia.HIV AIDS (Auckl). 2021 Jun 29;13:709-717. doi: 10.2147/HIV.S316776. eCollection 2021. HIV AIDS (Auckl). 2021. PMID: 34234572 Free PMC article.
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services; Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
-
- Wood E, Hogg RS, Yip B, Harrigan PR, Montaner JS. Why are baseline HIV RNA levels 100,000 copies/mL or greater associated with mortality after the initiation of antiretroviral therapy? J Acquir Immune Defic Syndr. 2005. March 1;38:289–95 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

