The genetic alteration spectrum of the SWI/SNF complex: The oncogenic roles of BRD9 and ACTL6A
- PMID: 31504061
- PMCID: PMC6736241
- DOI: 10.1371/journal.pone.0222305
The genetic alteration spectrum of the SWI/SNF complex: The oncogenic roles of BRD9 and ACTL6A
Abstract
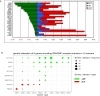
SWItch/Sucrose NonFermentable (SWI/SNF) is a set of multi-subunits chromatin remodeling complexes, playing important roles in a variety of biological processes. Loss-of-function mutations in the genes encoding SWI/SNF subunits have been reported in more than 20% of human cancers. Thus, it was widely considered as a tumor suppressor in the past decade. However, recent studies reported that some genes encoding subunits of SWI/SNF complexes were amplified and play oncogenic roles in human cancers. In present study, we summarized the genetic alteration spectrum of SWI/SNF complexes, and firstly systematically estimated both the copy number variations and point mutations of all 30 genes encoding the subunits in this complex. Additionally, the bioinformatics analyses were performed for two significantly amplified genes, ACTL6A and BRD9, to investigate their oncogenic roles in human cancers. Our findings may lay a foundation for the discovery of potential treatment targets in SWI/SNF complexes of cancers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Remodeling the cancer epigenome: mutations in the SWI/SNF complex offer new therapeutic opportunities.Expert Rev Anticancer Ther. 2019 May;19(5):375-391. doi: 10.1080/14737140.2019.1605905. Epub 2019 May 13. Expert Rev Anticancer Ther. 2019. PMID: 30986130 Free PMC article. Review.
-
BAFfling pathologies: Alterations of BAF complexes in cancer.Cancer Lett. 2018 Apr 10;419:266-279. doi: 10.1016/j.canlet.2018.01.046. Epub 2018 Jan 31. Cancer Lett. 2018. PMID: 29374542 Review.
-
ACTL6a enforces the epidermal progenitor state by suppressing SWI/SNF-dependent induction of KLF4.Cell Stem Cell. 2013 Feb 7;12(2):193-203. doi: 10.1016/j.stem.2012.12.014. Cell Stem Cell. 2013. PMID: 23395444 Free PMC article.
-
SWI/SNF chromatin remodeling and human malignancies.Annu Rev Pathol. 2015;10:145-71. doi: 10.1146/annurev-pathol-012414-040445. Epub 2014 Oct 27. Annu Rev Pathol. 2015. PMID: 25387058 Review.
-
The role of the SWI/SNF chromatin remodeling complex in pancreatic ductal adenocarcinoma.Cancer Sci. 2021 Feb;112(2):490-497. doi: 10.1111/cas.14768. Epub 2020 Dec 28. Cancer Sci. 2021. PMID: 33301642 Free PMC article. Review.
Cited by
-
Prognostic biomarker SMARCC1 and its association with immune infiltrates in hepatocellular carcinoma.Cancer Cell Int. 2021 Dec 22;21(1):701. doi: 10.1186/s12935-021-02413-w. Cancer Cell Int. 2021. PMID: 34937564 Free PMC article.
-
ACTL6A Promotes the Proliferation of Esophageal Squamous Cell Carcinoma Cells and Correlates with Poor Clinical Outcomes.Onco Targets Ther. 2021 Jan 13;14:199-211. doi: 10.2147/OTT.S288807. eCollection 2021. Onco Targets Ther. 2021. PMID: 33469301 Free PMC article.
-
GBAF, a small BAF sub-complex with big implications: a systematic review.Epigenetics Chromatin. 2020 Nov 3;13(1):48. doi: 10.1186/s13072-020-00370-8. Epigenetics Chromatin. 2020. PMID: 33143733 Free PMC article.
-
Chromatin remodellers as therapeutic targets.Nat Rev Drug Discov. 2024 Sep;23(9):661-681. doi: 10.1038/s41573-024-00978-5. Epub 2024 Jul 16. Nat Rev Drug Discov. 2024. PMID: 39014081 Free PMC article. Review.
-
BRD9 Degradation Disrupts Ribosome Biogenesis in Multiple Myeloma.Clin Cancer Res. 2023 May 1;29(9):1807-1821. doi: 10.1158/1078-0432.CCR-22-3668. Clin Cancer Res. 2023. PMID: 36780189 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

