Constructing functional cuticles: analysis of relationships between cuticle lipid composition, ultrastructure and water barrier function in developing adult maize leaves
- PMID: 31504131
- PMCID: PMC6948203
- DOI: 10.1093/aob/mcz143
Constructing functional cuticles: analysis of relationships between cuticle lipid composition, ultrastructure and water barrier function in developing adult maize leaves
Abstract
Background and aims: Prior work has examined cuticle function, composition and ultrastructure in many plant species, but much remains to be learned about how these features are related. This study aims to elucidate relationships between these features via analysis of cuticle development in adult maize (Zea mays L.) leaves, while also providing the most comprehensive investigation to date of the composition and ultrastructure of adult leaf cuticles in this important crop plant.
Methods: We examined water permeability, wax and cutin composition via gas chromatography, and ultrastructure via transmission electron microscopy, along the developmental gradient of partially expanded adult maize leaves, and analysed the relationships between these features.
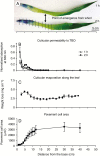
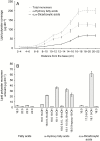
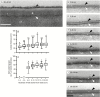
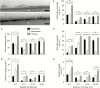
Key results: The water barrier property of the adult maize leaf cuticle is acquired at the cessation of cell expansion. Wax types and chain lengths accumulate asynchronously over the course of development, while overall wax load does not vary. Cutin begins to accumulate prior to establishment of the water barrier and continues thereafter. Ultrastructurally, pavement cell cuticles consist of an epicuticular layer, and a thin cuticle proper that acquires an inner, osmiophilic layer during development.
Conclusions: Cuticular waxes of the adult maize leaf are dominated by alkanes and alkyl esters. Unexpectedly, these are localized mainly in the epicuticular layer. Establishment of the water barrier during development coincides with a switch from alkanes to esters as the major wax type, and the emergence of an osmiophilic (likely cutin-rich) layer of the cuticle proper. Thus, alkyl esters and the deposition of the cutin polyester are implicated as key components of the water barrier property of adult maize leaf cuticles.
Keywords: Zea mays; Cuticular wax; cuticle ontogeny; cuticle ultrastructure; cutin; leaf development; maize.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures


Similar articles
-
Epicuticular wax on cherry laurel (Prunus laurocerasus) leaves does not constitute the cuticular transpiration barrier.Planta. 2016 Jan;243(1):65-81. doi: 10.1007/s00425-015-2397-y. Epub 2015 Sep 4. Planta. 2016. PMID: 26341347 Free PMC article.
-
Confocal laser scanning microscopy elucidation of the micromorphology of the leaf cuticle and analysis of its chemical composition.Protoplasma. 2015 Nov;252(6):1475-86. doi: 10.1007/s00709-015-0777-6. Epub 2015 Feb 25. Protoplasma. 2015. PMID: 25712592
-
ZmEREB46, a maize ortholog of Arabidopsis WAX INDUCER1/SHINE1, is involved in the biosynthesis of leaf epicuticular very-long-chain waxes and drought tolerance.Plant Sci. 2022 Aug;321:111256. doi: 10.1016/j.plantsci.2022.111256. Epub 2022 May 18. Plant Sci. 2022. PMID: 35696901
-
Cutinized and suberized barriers in leaves and roots: Similarities and differences.J Plant Physiol. 2023 Mar;282:153921. doi: 10.1016/j.jplph.2023.153921. Epub 2023 Jan 11. J Plant Physiol. 2023. PMID: 36780757 Review.
-
Protecting against water loss: analysis of the barrier properties of plant cuticles.J Exp Bot. 2001 Oct;52(363):2023-32. doi: 10.1093/jexbot/52.363.2023. J Exp Bot. 2001. PMID: 11559738 Review.
Cited by
-
The FUSED LEAVES1-ADHERENT1 regulatory module is required for maize cuticle development and organ separation.New Phytol. 2021 Jan;229(1):388-402. doi: 10.1111/nph.16837. Epub 2020 Aug 27. New Phytol. 2021. PMID: 32738820 Free PMC article.
-
Cutin:cutin-acid endo-transacylase (CCT), a cuticle-remodelling enzyme activity in the plant epidermis.Biochem J. 2021 Feb 26;478(4):777-798. doi: 10.1042/BCJ20200835. Biochem J. 2021. PMID: 33511979 Free PMC article.
-
Compositional variances in petal cuticular wax of eight rose species and their impacts on vase life under water-loss stress.Front Plant Sci. 2024 Sep 5;15:1412617. doi: 10.3389/fpls.2024.1412617. eCollection 2024. Front Plant Sci. 2024. PMID: 39301155 Free PMC article.
-
Cuticle development and the underlying transcriptome-metabolome associations during early seedling establishment.J Exp Bot. 2024 Oct 30;75(20):6500-6522. doi: 10.1093/jxb/erae311. J Exp Bot. 2024. PMID: 39031128 Free PMC article.
-
The Significance of Lipids for the Absorption and Release of Oxygen in Biological Organisms.Adv Exp Med Biol. 2023;1438:93-99. doi: 10.1007/978-3-031-42003-0_16. Adv Exp Med Biol. 2023. PMID: 37845446
References
-
- Avato P, Bianchi G, Pogna N. 1990. Chemosystematics of surface lipids from maize and some related species. Phytochemistry 29: 1571–1576.
-
- Avato P, Mikkelsen JD, von Wettstein-Knowles P. 1980. Effect of inhibitors on synthesis of fatty acyl chains present in waxes on developing maize leaves. Carlsberg Research Communications 45: 329–347.
-
- Baker EA. 1982. Chemistry and morphology of plant epicuticular waxes. In Cutler DF, Alvin KL, Price CE, eds. The plant cuticle. Linnean Society Symposium Series, Vol. 10 London: Academic Press, 139–165.
-
- Bargel H, Koch K, Cerman Z, Neinhuis C. 2006. Evans Review No. 3: Structure–function relationships of the plant cuticle and cuticular waxes – a smart material? Functional Plant Biology 33: 893–910. - PubMed
-
- Bianchi G, Avato P, Salamini F. 1978. Glossy mutants of maize. VIII. Accumulation of fatty aldehydes in surface waxes of gl5 maize seedlings. Biochemical Genetics 16: 1015–1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

