Friend or foe-IDH1 mutations in glioma 10 years on
- PMID: 31504231
- PMCID: PMC6875900
- DOI: 10.1093/carcin/bgz134
Friend or foe-IDH1 mutations in glioma 10 years on
Abstract
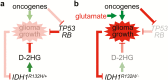
The identification of recurrent point mutations in the isocitrate dehydrogenase 1 (IDH1) gene, albeit in only a small percentage of glioblastomas a decade ago, has transformed our understanding of glioma biology, genomics and metabolism. More than 1000 scientific papers have been published since, propelling bench-to-bedside investigations that have led to drug development and clinical trials. The rapid biomedical advancement has been driven primarily by the realization of a neomorphic activity of IDH1 mutation that produces high levels of (d)-2-hydroxyglutarate, a metabolite believed to promote glioma initiation and progression through epigenetic and metabolic reprogramming. Thus, novel inhibitors of mutant IDH1 have been developed for therapeutic targeting. However, numerous clinical and experimental findings are at odds with this simple concept. By taking into consideration a large body of findings in the literature, this article analyzes how different approaches have led to opposing conclusions and proffers a counterintuitive hypothesis that IDH1 mutation is intrinsically tumor suppressive in glioma but functionally undermined by the glutamate-rich cerebral environment, inactivation of tumor-suppressor genes and IDH1 copy-number alterations. This theory also provides an explanation for some of the most perplexing observations, including the scarcity of proper model systems and the prevalence of IDH1 mutation in glioma.
© The Author(s) 2019. Published by Oxford University Press.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

