Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis
- PMID: 31504252
- PMCID: PMC7314637
- DOI: 10.1093/cvr/cvz238
Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis
Abstract
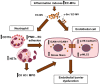
Aims: Microvesicles (MVs) conduct intercellular communication and impact diverse biological processes by transferring bioactive cargos to other cells. We investigated whether and how endothelial production of MVs contribute to vascular dysfunction during inflammation.
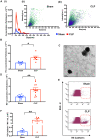
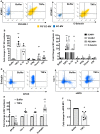
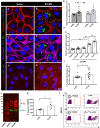
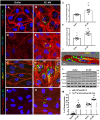
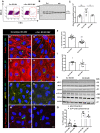
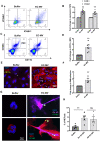
Methods and results: We measured the levels and molecular properties of endothelial-derived MVs (EC-MVs) from mouse plasma following a septic injury elicited by cecal ligation and puncture, as well as those from supernatants of cultured endothelial cells stimulated by inflammatory agents including cytokines, thrombin, and complement 5a. The mouse studies showed that sepsis caused a significant increase in total plasma vesicles and VE-cadherin+ EC-MVs compared to sham control. In cultured ECs, different inflammatory agents caused diverse patterns of EC-MV production and cargo contents. When topically applied to endothelial cells, EC-MVs induced a cytoskeleton-junction response characterized by myosin light chain phosphorylation, contractile fibre reorganization, VE-cadherin phosphorylation, and adherens junction dissociation, functionally measured as increased albumin transendothelial flux and decreased barrier resistance. The endothelial response was coupled with protein tyrosine phosphorylation promoted by MV cargo containing c-Src kinase, whereas MVs produced from c-Src deficient cells did not exert barrier-disrupting effects. Additionally, EC-MVs contribute to endothelial inflammatory injury by promoting neutrophil-endothelium adhesion and release of neutrophil extracellular traps containing citrullinated histones and myeloperoxidase, a response unaltered by c-Src knockdown.
Conclusion: Endothelial-derived microparticles cause endothelial barrier dysfunction by impairing adherens junctions and activating neutrophils. The signalling mechanisms underlying the endothelial cytoskeleton-junction response to EC-MVs involve protein phosphorylation promoted by MV cargo carrying c-Src. However, EC-MV-induced neutrophil activation was not dependent on c-Src.
Keywords: Barrier function; Endothelial cells; Inflammation; Microvesicles; Neutrophils.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

