Human iPSC modelling of a familial form of atrial fibrillation reveals a gain of function of If and ICaL in patient-derived cardiomyocytes
- PMID: 31504264
- PMCID: PMC7177512
- DOI: 10.1093/cvr/cvz217
Human iPSC modelling of a familial form of atrial fibrillation reveals a gain of function of If and ICaL in patient-derived cardiomyocytes
Abstract
Aims: Atrial fibrillation (AF) is the most common type of cardiac arrhythmias, whose incidence is likely to increase with the aging of the population. It is considered a progressive condition, frequently observed as a complication of other cardiovascular disorders. However, recent genetic studies revealed the presence of several mutations and variants linked to AF, findings that define AF as a multifactorial disease. Due to the complex genetics and paucity of models, molecular mechanisms underlying the initiation of AF are still poorly understood. Here we investigate the pathophysiological mechanisms of a familial form of AF, with particular attention to the identification of putative triggering cellular mechanisms, using patient's derived cardiomyocytes (CMs) differentiated from induced pluripotent stem cells (iPSCs).
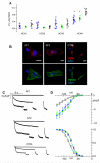
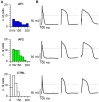
Methods and results: Here we report the clinical case of three siblings with untreatable persistent AF whose whole-exome sequence analysis revealed several mutated genes. To understand the pathophysiology of this multifactorial form of AF we generated three iPSC clones from two of these patients and differentiated these cells towards the cardiac lineage. Electrophysiological characterization of patient-derived CMs (AF-CMs) revealed that they have higher beating rates compared to control (CTRL)-CMs. The analysis showed an increased contribution of the If and ICaL currents. No differences were observed in the repolarizing current IKr and in the sarcoplasmic reticulum calcium handling. Paced AF-CMs presented significantly prolonged action potentials and, under stressful conditions, generated both delayed after-depolarizations of bigger amplitude and more ectopic beats than CTRL cells.
Conclusions: Our results demonstrate that the common genetic background of the patients induces functional alterations of If and ICaL currents leading to a cardiac substrate more prone to develop arrhythmias under demanding conditions. To our knowledge this is the first report that, using patient-derived CMs differentiated from iPSC, suggests a plausible cellular mechanism underlying this complex familial form of AF.
Keywords: Arrhythmias; Atrial fibrillation; Ion channels; Precision medicine; iPSC-derived cardiomyocytes.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures






Comment in
-
Atrial fibrillation in a dish: insights into atrial arrhythmogenesis from induced pluripotent stem cell-derived cardiomyocytes.Cardiovasc Res. 2020 May 1;116(6):1089-1091. doi: 10.1093/cvr/cvz342. Cardiovasc Res. 2020. PMID: 31860074 Free PMC article. No abstract available.

