The Clinical Profile of Severe Pediatric Malaria in an Area Targeted for Routine RTS,S/AS01 Malaria Vaccination in Western Kenya
- PMID: 31504308
- PMCID: PMC7353324
- DOI: 10.1093/cid/ciz844
The Clinical Profile of Severe Pediatric Malaria in an Area Targeted for Routine RTS,S/AS01 Malaria Vaccination in Western Kenya
Abstract
Background: The malaria prevalence has declined in western Kenya, resulting in the risk of neurological phenotypes in older children. This study investigates the clinical profile of pediatric malaria admissions ahead of the introduction of the RTS,S/AS01 vaccine.
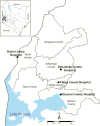
Methods: Malaria admissions in children aged 1 month to 15 years were identified from routine, standardized, inpatient clinical surveillance data collected between 2015 and 2018 from 4 hospitals in western Kenya. Malaria phenotypes were defined based on available data.
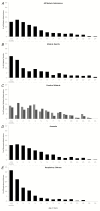
Results: There were 5766 malaria admissions documented. The median age was 36 months (interquartile range, 18-60): 15% were aged between 1-11 months of age, 33% were aged 1-23 months of age, and 70% were aged 1 month to 5 years. At admission, 2340 (40.6%) children had severe malaria: 421/2208 (19.1%) had impaired consciousness, 665/2240 (29.7%) had an inability to drink or breastfeed, 317/2340 (13.6%) had experienced 2 or more convulsions, 1057/2340 (45.2%) had severe anemia, and 441/2239 (19.7%) had severe respiratory distress. Overall, 211 (3.7%) children admitted with malaria died; 163/211 (77% deaths, case fatality rate 7.0%) and 48/211 (23% deaths, case fatality rate 1.4%) met the criteria for severe malaria and nonsevere malaria at admission, respectively. The median age for fatal cases was 33 months (interquartile range, 12-72) and the case fatality rate was highest in those unconscious (44.4%).
Conclusions: Severe malaria in western Kenya is still predominantly seen among the younger pediatric age group and current interventions targeted for those <5 years are appropriate. However, there are increasing numbers of children older than 5 years admitted with malaria, and ongoing hospital surveillance would identify when interventions should target older children.
Keywords: Kenya; admissions; children; malaria; severe.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Waiting for the Malaria Vaccine: The Complex Epidemiological Transition Toward Malaria Elimination.Clin Infect Dis. 2020 Jul 11;71(2):381-382. doi: 10.1093/cid/ciz847. Clin Infect Dis. 2020. PMID: 31504320 No abstract available.
References
-
- World Health Organization. World malaria report 2018 Available at: https://www.who.int/malaria/publications/world-malaria-report-2018/repor.... Accessed 11 March 2019.
-
- World Health Organization. Global technical strategy for malaria 2016–2030 Available at: https://apps.who.int/iris/bitstream/handle/10665/176712/9789241564991_en.... Accessed 11 March 2019.
-
- PMI. U.S. President’s Malaria Initiative Kenya: malaria operational plan FY 2018 Available at: https://www.pmi.gov/docs/default-source/default-document-library/malaria.... Accessed 11 March 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

