Potential for Point-of-Care Tests to Reduce Chlamydia-associated Burden in the United States: A Mathematical Modeling Analysis
- PMID: 31504314
- PMCID: PMC7048627
- DOI: 10.1093/cid/ciz519
Potential for Point-of-Care Tests to Reduce Chlamydia-associated Burden in the United States: A Mathematical Modeling Analysis
Abstract
Background: Point-of-care testing (POCT) assays for chlamydia are being developed. Their potential impact on the burden of chlamydial infection in the United States, in light of suboptimal screening coverage, remains unclear.
Methods: Using a transmission model calibrated to data in the United States, we estimated the impact of POCT on chlamydia prevalence, incidence, and chlamydia-attributable pelvic inflammatory disease (PID) incidence, assuming status quo (Analysis 1) and improved (Analysis 2) screening frequencies. We tested the robustness of results to changes in POCT sensitivity, the proportion of patients getting treated immediately, the baseline proportion lost to follow-up (LTFU), and the average treatment delay.
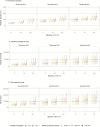
Results: In Analysis 1, high POCT sensitivity was needed to reduce the chlamydia-associated burden. With a POCT sensitivity of 90%, reductions from the baseline burden only occurred in scenarios in which over 60% of the screened individuals would get immediate treatment and the baseline LTFU proportion was 20%. With a POCT sensitivity of 99% (baseline LTFU 10%, 2-week treatment delay), if everyone were treated immediately, the prevalence reduction was estimated at 5.7% (95% credible interval [CrI] 3.9-8.2%). If only 30% of tested persons would wait for results, the prevalence reduction was only 1.6% (95% CrI 1.1-2.3). POCT with 99% sensitivity could avert up to 12 700 (95% CrI 5000-22 200) PID cases per year, if 100% were treated immediately (baseline LTFU 20% and 3-week treatment delay). In Analysis 2, when POCT was coupled with increasing screening coverage, reductions in the chlamydia burden could be realized with a POCT sensitivity of 90%.
Conclusions: POCT could improve chlamydia prevention efforts if test performance characteristics are significantly improved over currently available options.
Keywords: chlamydia; diagnostics; mathematical model; point-of-care; screening.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Making the Most of Point-of-care Testing for Sexually Transmitted Diseases.Clin Infect Dis. 2020 Apr 15;70(9):1824-1825. doi: 10.1093/cid/ciz523. Clin Infect Dis. 2020. PMID: 31504333 No abstract available.
References
-
- World Health Organization. Point-of-care diagnostic tests (POCTs) for sexually transmitted infections (STIs). Geneva, Switzerland: World Health Organization, 2017. Available at: http://www.who.int/reproductivehealth/topics/rtis/pocts/en/. Accessed 31 March 2018.
-
- Gift TL, Pate MS, Hook EW 3rd, Kassler WJ. The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for Chlamydia trachomatis. Sex Transm Dis 1999; 26:232–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

