Tissue-Specific Ablation of ACSL4 Results in Disturbed Steroidogenesis
- PMID: 31504388
- PMCID: PMC6773434
- DOI: 10.1210/en.2019-00464
Tissue-Specific Ablation of ACSL4 Results in Disturbed Steroidogenesis
Abstract
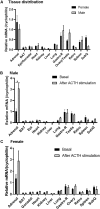
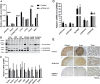
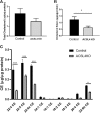
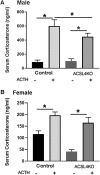
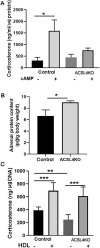
ACSL4 is a member of the ACSL family that catalyzes the conversion of long-chain fatty acids to acyl-coenzyme As, which are essential for fatty-acid incorporation and utilization in diverse metabolic pathways, including cholesteryl ester synthesis. Steroidogenic tissues such as the adrenal gland are particularly enriched in cholesteryl esters of long-chain polyunsaturated fatty acids, which constitute an important pool supplying cholesterol for steroid synthesis. The current studies addressed whether ACSL4 is required for normal steroidogenesis. CYP11A1 promoter‒mediated Cre was used to generate steroid tissue‒specific ACSL4 knockout (KO) mice. Results demonstrated that ACSL4 plays an important role in adrenal cholesteryl ester formation, as well as in determining the fatty acyl composition of adrenal cholesteryl esters, with ACSL4 deficiency leading to reductions in cholesteryl ester storage and alterations in cholesteryl ester composition. Statistically significant reductions in corticosterone and testosterone production, but not progesterone production, were observed in vivo, and these deficits were accentuated in ex vivo and in vitro studies of isolated steroid tissues and cells from ACSL4-deficient mice. However, these effects on steroid production appear to be due to reductions in cholesteryl ester stores rather than disturbances in signaling pathways. We conclude that ACSL4 is dispensable for normal steroidogenesis.
Published by Oxford University Press on behalf of the Endocrine Society 2019.
Figures
Similar articles
-
A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction.Mol Metab. 2018 Mar;9:43-56. doi: 10.1016/j.molmet.2018.01.012. Epub 2018 Jan 31. Mol Metab. 2018. PMID: 29398618 Free PMC article.
-
Long-chain acyl-CoA synthetase 4 participates in the formation of highly unsaturated fatty acid-containing phospholipids in murine macrophages.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Nov;1864(11):1606-1618. doi: 10.1016/j.bbalip.2019.07.013. Epub 2019 Jul 31. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 31376475
-
Long-chain acyl-CoA synthetase 4 is regulated by phosphorylation.Biochem Biophys Res Commun. 2013 Jan 4;430(1):272-7. doi: 10.1016/j.bbrc.2012.10.138. Epub 2012 Nov 15. Biochem Biophys Res Commun. 2013. PMID: 23159612
-
Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism.Prostaglandins Other Lipid Mediat. 2019 Oct;144:106363. doi: 10.1016/j.prostaglandins.2019.106363. Epub 2019 Jul 12. Prostaglandins Other Lipid Mediat. 2019. PMID: 31306767 Review.
-
Adrenal cholesterol utilization.Mol Cell Endocrinol. 2007 Feb;265-266:42-5. doi: 10.1016/j.mce.2006.12.001. Epub 2007 Jan 8. Mol Cell Endocrinol. 2007. PMID: 17208360 Review.
Cited by
-
ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics.Front Pharmacol. 2022 Jul 13;13:949863. doi: 10.3389/fphar.2022.949863. eCollection 2022. Front Pharmacol. 2022. PMID: 35910359 Free PMC article. Review.
-
ACSL4: biomarker, mediator and target in quadruple negative breast cancer.Oncotarget. 2023 Jun 12;14:563-575. doi: 10.18632/oncotarget.28453. Oncotarget. 2023. PMID: 37306503 Free PMC article. Review.
-
The ACSL4 Network Regulates Cell Death and Autophagy in Diseases.Biology (Basel). 2023 Jun 15;12(6):864. doi: 10.3390/biology12060864. Biology (Basel). 2023. PMID: 37372148 Free PMC article. Review.
-
Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1.Nat Commun. 2021 Apr 14;12(1):2244. doi: 10.1038/s41467-021-22471-y. Nat Commun. 2021. PMID: 33854057 Free PMC article.
-
Mice deficient in ER protein seipin have reduced adrenal cholesteryl ester lipid droplet formation and utilization.J Lipid Res. 2022 Dec;63(12):100309. doi: 10.1016/j.jlr.2022.100309. Epub 2022 Nov 1. J Lipid Res. 2022. PMID: 36332685 Free PMC article.
References
-
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. - PubMed
-
- Hiort O, Holterhus PM, Nitsche EM. Physiology and pathophysiology of androgen action. Baillieres Clin Endocrinol Metab. 1998;12(1):115–132. - PubMed
-
- Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74(5):279–285. - PubMed
-
- Pearce D, Bhargava A, Cole TJ. Aldosterone: its receptor, target genes, and actions. Vitam Horm. 2003;66:29–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

