Reduced Stability and pH-Dependent Activity of a Common Obesity-Linked PCSK1 Polymorphism, N221D
- PMID: 31504391
- PMCID: PMC6892424
- DOI: 10.1210/en.2019-00418
Reduced Stability and pH-Dependent Activity of a Common Obesity-Linked PCSK1 Polymorphism, N221D
Abstract
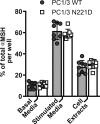
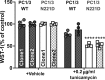
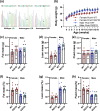
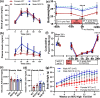
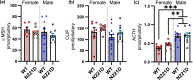
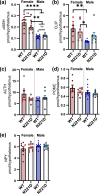
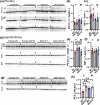
Common mutations in the human prohormone convertase (PC)1/3 gene (PCKSI) are linked to increased risk of obesity. Previous work has shown that the rs6232 single-nucleotide polymorphism (N221D) results in slightly decreased activity, although whether this decrease underlies obesity risk is not clear. We observed significantly decreased activity of the N221D PC1/3 enzyme at the pH of the trans-Golgi network; at this pH, the mutant enzyme was less stable than wild-type enzyme. Recombinant N221D PC1/3 also showed enhanced susceptibility to heat stress. Enhanced susceptibility to tunicamycin-induced endoplasmic reticulum stress was observed in AtT-20/PC2 cell clones in which murine PC1/3 was replaced by human N221D PC1/3, as compared with wild-type human PC1/3. However, N221D PC1/3-expressing AtT-20/PC2 clones processed proopiomelanocortin to α-MSH similarly to wild-type PC1/3. We also generated a CRISPR-edited mouse line expressing the N221D mutation in the PCKSI gene. When homozygous N221D mice were fed either a standard or a high-fat diet, we found no increase in body weight compared with their wild-type sibling controls. Sexual dimorphism was observed in pituitary ACTH for both genotypes, with females exhibiting lower levels of pituitary ACTH. In contrast, hypothalamic α-MSH content for both genotypes was higher in females compared with males. Hypothalamic corticotropin-like intermediate peptide content was higher in wild-type females compared with wild-type, but not N221D, males. Taken together, these data suggest that the increased obesity risk linked to the N221D allele in humans may be due in part to PC1/3-induced loss of resilience to stressors rather than strictly to decreased enzymatic activity on peptide precursors.
Copyright © 2019 Endocrine Society.
Figures
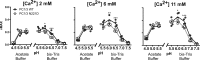

Similar articles
-
Endoplasmic reticulum-associated degradation of the mouse PC1/3-N222D hypomorph and human PCSK1 mutations contributes to obesity.Int J Obes (Lond). 2016 Jun;40(6):973-81. doi: 10.1038/ijo.2016.3. Epub 2016 Jan 20. Int J Obes (Lond). 2016. PMID: 26786350
-
Revisiting PC1/3 Mutants: Dominant-Negative Effect of Endoplasmic Reticulum-Retained Mutants.Endocrinology. 2015 Oct;156(10):3625-37. doi: 10.1210/en.2015-1068. Epub 2015 Jul 24. Endocrinology. 2015. PMID: 26207343 Free PMC article.
-
Effects of rs6234/rs6235 and rs6232/rs6234/rs6235 PCSK1 single-nucleotide polymorphism clusters on proprotein convertase 1/3 biosynthesis and activity.Mol Genet Metab. 2011 Dec;104(4):682-7. doi: 10.1016/j.ymgme.2011.09.027. Epub 2011 Sep 24. Mol Genet Metab. 2011. PMID: 22000902
-
PCSK1 Variants and Human Obesity.Prog Mol Biol Transl Sci. 2016;140:47-74. doi: 10.1016/bs.pmbts.2015.12.001. Epub 2016 Jan 29. Prog Mol Biol Transl Sci. 2016. PMID: 27288825 Free PMC article. Review.
-
The subtilisin/kexin family of precursor convertases. Emphasis on PC1, PC2/7B2, POMC and the novel enzyme SKI-1.Ann N Y Acad Sci. 1999 Oct 20;885:57-74. doi: 10.1111/j.1749-6632.1999.tb08665.x. Ann N Y Acad Sci. 1999. PMID: 10816641 Review.
Cited by
-
Association between SNPs in Leptin Pathway Genes and Anthropometric, Biochemical, and Dietary Markers Related to Obesity.Genes (Basel). 2022 May 25;13(6):945. doi: 10.3390/genes13060945. Genes (Basel). 2022. PMID: 35741707 Free PMC article.
-
Mice lacking PC1/3 expression in POMC-expressing cells do not develop obesity.Endocrinology. 2021 Mar 10;162(6):bqab055. doi: 10.1210/endocr/bqab055. Online ahead of print. Endocrinology. 2021. PMID: 33693631 Free PMC article.
-
Obesity, POMC, and POMC-processing Enzymes: Surprising Results From Animal Models.Endocrinology. 2021 Dec 1;162(12):bqab155. doi: 10.1210/endocr/bqab155. Endocrinology. 2021. PMID: 34333593 Free PMC article. Review.
-
Mouse Models of Human Proprotein Convertase Insufficiency.Endocr Rev. 2021 May 25;42(3):259-294. doi: 10.1210/endrev/bnaa033. Endocr Rev. 2021. PMID: 33382413 Free PMC article. Review.
-
The G209R mutant mouse as a model for human PCSK1 polyendocrinopathy.Endocrinology. 2022 Mar 4;163(5):bqac024. doi: 10.1210/endocr/bqac024. Online ahead of print. Endocrinology. 2022. PMID: 35245347 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

