Toll-Like Receptor-4 Antagonist (+)-Naloxone Confers Sexually Dimorphic Protection From Inflammation-Induced Fetal Programming in Mice
- PMID: 31504393
- PMCID: PMC6936318
- DOI: 10.1210/en.2019-00493
Toll-Like Receptor-4 Antagonist (+)-Naloxone Confers Sexually Dimorphic Protection From Inflammation-Induced Fetal Programming in Mice
Abstract
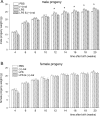
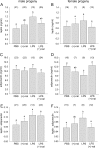
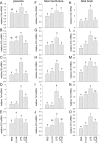
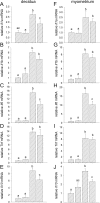
Inflammation elicited by infection or noninfectious insults during gestation induces proinflammatory cytokines that can shift the trajectory of development to alter offspring phenotype, promote adiposity, and increase susceptibility to metabolic disease in later life. In this study, we use mice to investigate the utility of a small molecule Toll-like receptor (TLR)4 antagonist (+)-naloxone, the nonopioid isomer of the opioid receptor antagonist (-)-naloxone, for mitigating altered fetal metabolic programming induced by a modest systemic inflammatory challenge in late gestation. In adult progeny exposed to lipopolysaccharide (LPS) challenge in utero, male but not female offspring exhibited elevated adipose tissue, reduced muscle mass, and elevated plasma leptin at 20 weeks of age. Effects were largely reversed by coadministration of (+)-naloxone following LPS. When given alone without LPS, (+)-naloxone elicited accelerated postweaning growth and elevated muscle and fat mass in adult male but not female offspring. LPS induced expression of inflammatory cytokines Il1a, Il1b, Il6, Tnf, and Il10 in fetal brain, placental, and uterine tissues, and (+)-naloxone suppressed LPS-induced cytokine expression. Fetal sex-specific regulation of cytokine expression was evident, with higher Il1a, Il1b, Il6, and Il10 induced by LPS in tissues associated with male fetuses, and greater suppression by (+)-naloxone of Il6 in females. These data demonstrate that modulating TLR4 signaling with (+)-naloxone provides protection from inflammatory diversion of fetal developmental programming in utero, associated with attenuation of gestational tissue cytokine expression in a fetal sex-specific manner. The results suggest that pharmacologic interventions targeting TLR4 warrant evaluation for attenuating developmental programming effects of fetal exposure to maternal inflammatory mediators.
Copyright © 2019 Endocrine Society.
Figures

Similar articles
-
Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury.Clin Transl Immunology. 2020 Apr 14;9(4):e1121. doi: 10.1002/cti2.1121. eCollection 2020 Apr. Clin Transl Immunology. 2020. PMID: 32313651 Free PMC article. Review.
-
Novel Toll-like receptor-4 antagonist (+)-naloxone protects mice from inflammation-induced preterm birth.Sci Rep. 2016 Nov 7;6:36112. doi: 10.1038/srep36112. Sci Rep. 2016. PMID: 27819333 Free PMC article.
-
Toll-Like Receptor 4 Is an Essential Upstream Regulator of On-Time Parturition and Perinatal Viability in Mice.Endocrinology. 2015 Oct;156(10):3828-41. doi: 10.1210/EN.2015-1089. Epub 2015 Jul 7. Endocrinology. 2015. PMID: 26151355 Free PMC article.
-
Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice.Biol Reprod. 2007 May;76(5):738-48. doi: 10.1095/biolreprod.106.056143. Epub 2007 Jan 10. Biol Reprod. 2007. PMID: 17215490
-
Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease.Int J Mol Sci. 2021 Feb 9;22(4):1732. doi: 10.3390/ijms22041732. Int J Mol Sci. 2021. PMID: 33572203 Free PMC article. Review.
Cited by
-
Effects of prenatal opioid and alcohol exposures on immune and serotonin factors in human placenta.Exp Neurol. 2022 Jul;353:114057. doi: 10.1016/j.expneurol.2022.114057. Epub 2022 Mar 29. Exp Neurol. 2022. PMID: 35364108 Free PMC article.
-
Effects of Toll-like receptor 4 inhibition on spatial memory and cell proliferation in male and female adult and aged mice.Brain Behav Immun. 2021 Oct;97:383-393. doi: 10.1016/j.bbi.2021.06.008. Epub 2021 Jul 31. Brain Behav Immun. 2021. PMID: 34343615 Free PMC article.
-
The Influence of Maternal High Fat Diet During Lactation on Offspring Hematopoietic Priming.Endocrinology. 2023 Nov 20;165(1):bqad182. doi: 10.1210/endocr/bqad182. Endocrinology. 2023. PMID: 38048597 Free PMC article.
-
Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury.Clin Transl Immunology. 2020 Apr 14;9(4):e1121. doi: 10.1002/cti2.1121. eCollection 2020 Apr. Clin Transl Immunology. 2020. PMID: 32313651 Free PMC article. Review.
-
LPS versus Poly I:C model: comparison of long-term effects of bacterial and viral maternal immune activation on the offspring.Am J Physiol Regul Integr Comp Physiol. 2022 Feb 1;322(2):R99-R111. doi: 10.1152/ajpregu.00087.2021. Epub 2021 Dec 7. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 34874190 Free PMC article. Review.
References
-
- Ozanne SE, Constância M. Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab. 2007;3(7):539–546. - PubMed
-
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21(4):199–205. - PubMed
-
- Hui LL, Lam HS, Leung GM, Schooling CM. Late prematurity and adiposity in adolescents: evidence from “Children of 1997” birth cohort. Obesity (Silver Spring). 2015;23(11):2309–2314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

