Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review
- PMID: 31504506
- PMCID: PMC6737562
- DOI: 10.1093/humupd/dmz024
Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review
Abstract
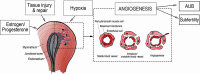
Background: Adenomyosis commonly occurs with abnormal uterine bleeding (AUB) and is associated with subfertility and a higher miscarriage rate. Recent evidence showed abnormal vascularization in the endometrium in patients with adenomyosis, suggesting a role of angiogenesis in the pathophysiology of AUB and subfertility in adenomyosis and providing a possible treatment target.
Objective and rationale: We hypothesized that the level of abnormal vascularization and expression of angiogenic markers is increased in the ectopic and eutopic endometrium of adenomyosis patients in comparison with the endometrium of control patients. This was investigated through a search of the literature.
Search methods: A systematic search was performed in PubMed and Embase until February 2019. Combinations of terms for angiogenesis and adenomyosis were applied as well as AUB, subfertility or anti-angiogenic therapy. The main search was limited to clinical studies carried out on premenopausal women. Original research articles focusing on markers of angiogenesis in the endometrium of patients with adenomyosis were included. Studies in which no comparison was made to control patients or which were not published in a peer-reviewed journal were excluded. A second search was performed to explore the therapeutic potential of targeting angiogenesis in adenomyosis. This search also included preclinical studies.
Outcomes: A total of 20 articles out of 1669 hits met our selection criteria. The mean vascular density (MVD) was studied by quantification of CD31, CD34, von Willebrand Factor (vWF) or factor-VIII-antibody-stained microvessels in seven studies. All these studies reported a significantly increased MVD in ectopic endometrium, and out of the six articles that took it into account, four studies reported a significantly increased MVD in eutopic endometrium compared with control endometrium. Five articles showed a significantly higher vascular endothelial growth factor expression in ectopic endometrium and three articles in eutopic endometrium compared with control endometrium. The vascular and pro-angiogenic markers α-smooth muscle actin, endoglin, S100A13, vimentin, matrix metalloproteinases (MMPs), nuclear factor (NF)-kB, tissue factor (TF), DJ-1, phosphorylated mammalian target of rapamycin, activin A, folli- and myostatin, CD41, SLIT, roundabout 1 (ROBO1), cyclooxygenase-2, lysophosphatidic acid (LPA) 1,4-5, phospho signal transducer and activator of transcription 3 (pSTAT3), interleukin (IL)-6, IL-22 and transforming growth factor-β1 were increased in ectopic endometrium, and the markers S100A13, MMP-2 and -9, TF, follistatin, myostatin, ROBO1, LPA1 and 4-5, pSTAT3, IL-6 and IL-22 were increased in eutopic endometrium, compared with control endometrium. The anti-angiogenic markers E-cadherin, eukaryotic translation initiation factor 3 subunit and gene associated with retinoic-interferon-induced mortality 19 were decreased in ectopic endometrium and IL-10 in eutopic endometrium, compared with control endometrium. The staining level of vWF and two pro-angiogenic markers (NF-κB nuclear p65 and TF) correlated with AUB in patients with adenomyosis. We found no studies that investigated the possible relationship between markers of angiogenesis and subfertility in adenomyosis patients. Nine articles reported on direct or indirect targeting of angiogenesis in adenomyosis-either by testing hormonal therapy or herbal compounds in clinical studies or by testing angiogenesis inhibitors in preclinical studies. However, there are no clinical studies on the effectiveness of such therapy for adenomyosis-related AUB or subfertility.
Wider implications: The results are in agreement with our hypothesis that increased angiogenesis is present in the endometrium of patients with adenomyosis compared with the endometrium of control patients. It is likely that increased angiogenesis leads to fragile and more permeable vessels resulting in adenomyosis-related AUB and possibly subfertility. While this association has not sufficiently been studied yet, our results encourage future studies to investigate the exact role of angiogenesis in the etiology of adenomyosis and related AUB or subfertility in women with adenomyosis in order to design curative or preventive therapeutic strategies.
Keywords: abnormal uterine bleeding; adenomyosis; angiogenesis; anti-angiogenic therapy; endometriosis; subfertility.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures
Similar articles
-
Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry.Int J Mol Sci. 2022 Jul 29;23(15):8434. doi: 10.3390/ijms23158434. Int J Mol Sci. 2022. PMID: 35955568 Free PMC article.
-
Angiogenesis in abnormal uterine bleeding: a narrative review.Hum Reprod Update. 2023 Jul 5;29(4):457-485. doi: 10.1093/humupd/dmad004. Hum Reprod Update. 2023. PMID: 36857162 Free PMC article. Review.
-
Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis.Hum Reprod. 2016 Apr;31(4):734-49. doi: 10.1093/humrep/dew018. Epub 2016 Feb 22. Hum Reprod. 2016. PMID: 26908845
-
Immunological changes associated with adenomyosis: a systematic review.Hum Reprod Update. 2021 Jan 4;27(1):108-129. doi: 10.1093/humupd/dmaa038. Hum Reprod Update. 2021. PMID: 33099635
-
Transforming growth factor β1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice.Hum Reprod. 2016 Feb;31(2):355-69. doi: 10.1093/humrep/dev314. Epub 2015 Dec 20. Hum Reprod. 2016. PMID: 26689216
Cited by
-
CD36 and Its Role in Regulating the Tumor Microenvironment.Curr Oncol. 2022 Oct 27;29(11):8133-8145. doi: 10.3390/curroncol29110642. Curr Oncol. 2022. PMID: 36354702 Free PMC article. Review.
-
Physiomimetic Models of Adenomyosis.Semin Reprod Med. 2020 May;38(2-03):179-196. doi: 10.1055/s-0040-1719084. Epub 2020 Nov 9. Semin Reprod Med. 2020. PMID: 33176387 Free PMC article. Review.
-
Research Advances in Adenomyosis-Related Signaling Pathways and Promising Targets.Biomolecules. 2024 Nov 4;14(11):1402. doi: 10.3390/biom14111402. Biomolecules. 2024. PMID: 39595579 Free PMC article. Review.
-
Bioinformatic analysis and machine learning to identify the diagnostic biomarkers and immune infiltration in adenomyosis.Front Genet. 2023 Jan 4;13:1082709. doi: 10.3389/fgene.2022.1082709. eCollection 2022. Front Genet. 2023. PMID: 36685847 Free PMC article.
-
Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry.Int J Mol Sci. 2022 Jul 29;23(15):8434. doi: 10.3390/ijms23158434. Int J Mol Sci. 2022. PMID: 35955568 Free PMC article.
References
-
- Abberton KM, Healy DL, Rogers PA. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod 1999;14:3095–3100. - PubMed
-
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)-pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol 2017;40:68–81. - PubMed
-
- An M, Li D, Yuan M, Li Q, Zhang L, Wang G. Different macrophages equally induce EMT in endometria of adenomyosis and normal. Reproduction 2017;154:79–92. - PubMed
-
- Bani M, Decio A, Giavazzi R, Ghilardi C. Contribution of tumor endothelial cells to drug resistance: anti-angiogenic tyrosine kinase inhibitors act as p-glycoprotein antagonists. Angiogenesis 2017;20:233–241. - PubMed
-
- Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev 2015;67:441–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

