The history of IgG glycosylation and where we are now
- PMID: 31504525
- PMCID: PMC7109348
- DOI: 10.1093/glycob/cwz065
The history of IgG glycosylation and where we are now
Abstract
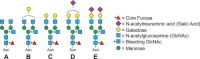
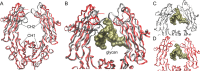
IgG glycosylation is currently at the forefront of both immunology and glycobiology, likely due in part to the widespread and growing use of antibodies as drugs. For over four decades, it has been recognized that the conserved N-linked glycan on asparagine 297 found within the second Ig domain of the heavy chain (CH2) that helps to comprise Fc region of IgG plays a special role in IgG structure and function. Changes in galactosylation, fucosylation and sialylation are now well-established factors, which drive differential IgG function, ranging from inhibitory/anti-inflammatory to activating complement and promoting antibody-dependent cellular cytotoxicity. Thus, if we are to truly understand how to design and deploy antibody-based drugs with maximal efficacy and evaluate proper vaccine responses from a protective and functional perspective, a deep understanding of IgG glycosylation is essential. This article is intended to provide a comprehensive review of the IgG glycosylation field and the impact glycans have on IgG function, beginning with the earliest findings over 40 years ago, in order to provide a robust foundation for moving forward.
Keywords: IgG; glycan; glycosylation; sialylation.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms.MAbs. 2019 Feb/Mar;11(2):350-372. doi: 10.1080/19420862.2018.1551044. Epub 2018 Dec 10. MAbs. 2019. PMID: 30466347 Free PMC article.
-
An atomistic perspective on antibody-dependent cellular cytotoxicity quenching by core-fucosylation of IgG1 Fc N-glycans from enhanced sampling molecular dynamics.Glycobiology. 2020 May 19;30(6):407-414. doi: 10.1093/glycob/cwz101. Glycobiology. 2020. PMID: 31829411
-
Modulating IgG effector function by Fc glycan engineering.Proc Natl Acad Sci U S A. 2017 Mar 28;114(13):3485-3490. doi: 10.1073/pnas.1702173114. Epub 2017 Mar 13. Proc Natl Acad Sci U S A. 2017. PMID: 28289219 Free PMC article.
-
A synopsis of recent developments defining how N-glycosylation impacts immunoglobulin G structure and function.Glycobiology. 2020 Mar 20;30(4):214-225. doi: 10.1093/glycob/cwz068. Glycobiology. 2020. PMID: 31822882 Free PMC article. Review.
-
IgG N-glycans.Adv Clin Chem. 2021;105:1-47. doi: 10.1016/bs.acc.2021.02.001. Epub 2021 Mar 18. Adv Clin Chem. 2021. PMID: 34809825 Review.
Cited by
-
Host glycosylation of immunoglobulins impairs the immune response to acute Lyme disease.EBioMedicine. 2024 Feb;100:104979. doi: 10.1016/j.ebiom.2024.104979. Epub 2024 Jan 23. EBioMedicine. 2024. PMID: 38266555 Free PMC article.
-
Enhanced proteomic profiling of human plasma-derived extracellular vesicles through charge-based fractionation to advance biomarker discovery potential.J Extracell Vesicles. 2024 Dec;13(12):e70024. doi: 10.1002/jev2.70024. J Extracell Vesicles. 2024. PMID: 39641316 Free PMC article.
-
The Association Between Neonatal Respiratory Distress Syndrome and Plasma IgG N-Glycosylation: A Case-Control Study.J Inflamm Res. 2025 May 21;18:6439-6451. doi: 10.2147/JIR.S524188. eCollection 2025. J Inflamm Res. 2025. PMID: 40416708 Free PMC article.
-
Oligomannose N-Glycans 3D Architecture and Its Response to the FcγRIIIa Structural Landscape.J Phys Chem B. 2021 Mar 18;125(10):2607-2616. doi: 10.1021/acs.jpcb.1c00304. Epub 2021 Mar 4. J Phys Chem B. 2021. PMID: 33661628 Free PMC article.
-
Antibodies as clinical tools for tuberculosis.Front Immunol. 2023 Dec 14;14:1278947. doi: 10.3389/fimmu.2023.1278947. eCollection 2023. Front Immunol. 2023. PMID: 38162666 Free PMC article. Review.
References
-
- Adler Y, Lamour A, Jamin C, Menez JF, Le Corre R, Shoenfeld Y, Youinou P. 1995. Impaired binding capacity of asialyl and agalactosyl IgG to Fc gamma receptors. Clin Exp Rheumatol. 13:315–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

