Corticosteroid-Free Remission vs Overall Remission in Clinical Trials of Moderate-Severe Ulcerative Colitis and Crohn's Disease
- PMID: 31504528
- PMCID: PMC8127062
- DOI: 10.1093/ibd/izz193
Corticosteroid-Free Remission vs Overall Remission in Clinical Trials of Moderate-Severe Ulcerative Colitis and Crohn's Disease
Abstract
Background: We summarized the protocol-specified corticosteroid tapering regimens in clinical trials of moderate-severe ulcerative colitis (UC) and Crohn's disease (CD) and calculated differences in rates of clinical remission vs corticosteroid-free clinical remission (CSF-CR).
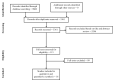
Methods: Through a systematic literature review through February 28, 2019, we identified 16 randomized controlled trials (RCTs) of biologics or small molecules in patients with moderate-severe UC or CD who reported CSF-CR as an outcome. We estimated the relative risk and 95% confidence interval of achieving CSF-CR vs overall clinical remission in patients treated with active intervention or placebo through random-effects meta-analysis.
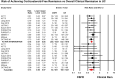
Results: Across trials of UC (11 trials) and CD (5 trials), a median of 53% and 49% of participants were on corticosteroids at the time of trial entry, respectively. Participants were allowed to enter trials at a median corticosteroid dose (range) of 35 (20-40) mg/d. Doses were kept stable for a median (range) of 8 (5-10) weeks during induction therapy, after which a mandatory and structured taper was implemented, albeit with the investigators' discretion depending on clinical status. Pooled rates of CSF-CR in patients with UC and CD treated with placebo were 9.7% and 19.1%, respectively. In UC and CD trials, the rate of CSF-CR was 24% and 18% lower than the rate of overall clinical remission, respectively.
Conclusions: Protocol-specified corticosteroid tapering regimens vary across trials. These findings will help to inform the design and interpretation of future clinical trials and highlight the need for standardization.
Keywords: Crohn’s disease; clinical trials; end points; maintenance therapy; ulcerative colitis.
© 2019 Crohn’s & Colitis Foundation. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Huscher D, Thiele K, Gromnica-Ihle E, et al. . Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68:1119–1124. - PubMed
-
- Ma C, Hussein IM, Al-Abbar YJ, et al. . Heterogeneity in definitions of efficacy and safety endpoints for clinical trials of Crohn’s disease: a systematic review. Clin Gastroenterol Hepatol. 2018;16:1407–1419.e22. - PubMed
-
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. - PubMed
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

