A Recombinant Chlamydia trachomatis MOMP Vaccine Elicits Cross-serogroup Protection in Mice Against Vaginal Shedding and Infertility
- PMID: 31504647
- PMCID: PMC6935996
- DOI: 10.1093/infdis/jiz438
A Recombinant Chlamydia trachomatis MOMP Vaccine Elicits Cross-serogroup Protection in Mice Against Vaginal Shedding and Infertility
Abstract
Background: Chlamydia trachomatis is the most common sexually transmitted bacterial pathogen worldwide. Here, we determined the ability of a C. trachomatis recombinant major outer membrane protein (rMOMP) vaccine to elicit cross-serogroup protection.
Methods: Female C3H/HeN mice were vaccinated by mucosal and systemic routes with C. trachomatis serovar D (UW-3/Cx) rMOMP and challenged in the ovarian bursa with serovars D (UW-3/Cx), D (UCI-96/Cx), E (IOL-43), or F (N.I.1). CpG-1826 and Montanide ISA 720 were used as adjuvants.
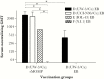
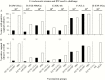
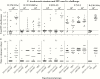
Results: Immune responses following vaccination were more robust against the most closely related serovars. Following a genital challenge (as determined by number of mice with positive vaginal cultures, number of positive cultures, number of inclusion forming units recovered, and number of days with positive cultures) mice challenged with C. trachomatis serovars of the same complex were protected but not those challenged with serovar F (N.I.1) from a different subcomplex. Females were caged with male mice. Based on fertility rates, number of embryos, and hydrosalpinx formation, vaccinated mice were protected against challenges with serovars D (UW-3/Cx), D (UCI-96/Cx), and E (IOL-43) but not F (N.I.1).
Conclusions: This is the first subunit vaccine shown to protect mice against infection, pathology, and infertility caused by different C. trachomatis serovars.
Keywords: Chlamydia trachomatis; MOMP; cross-serogroup; mice; protection; vaccine.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Vaccination with the recombinant major outer membrane protein elicits antibodies to the constant domains and induces cross-serovar protection against intranasal challenge with Chlamydia trachomatis.Infect Immun. 2013 May;81(5):1741-50. doi: 10.1128/IAI.00734-12. Epub 2013 Mar 11. Infect Immun. 2013. PMID: 23478318 Free PMC article.
-
Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria.Infect Immun. 2005 Dec;73(12):8153-60. doi: 10.1128/IAI.73.12.8153-8160.2005. Infect Immun. 2005. PMID: 16299310 Free PMC article.
-
A vaccine formulated with the major outer membrane protein can protect C3H/HeN, a highly susceptible strain of mice, from a Chlamydia muridarum genital challenge.Immunology. 2015 Nov;146(3):432-43. doi: 10.1111/imm.12520. Epub 2015 Oct 1. Immunology. 2015. PMID: 26423798 Free PMC article.
-
Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development.J Reprod Immunol. 2000 Aug;48(1):47-68. doi: 10.1016/s0165-0378(00)00069-3. J Reprod Immunol. 2000. PMID: 10996382 Review.
-
The mucosal immune response to Chlamydia trachomatis infection of the reproductive tract in women.J Reprod Immunol. 2009 Dec;83(1-2):173-8. doi: 10.1016/j.jri.2009.07.013. Epub 2009 Nov 5. J Reprod Immunol. 2009. PMID: 19896206 Review.
Cited by
-
Immune signature of Chlamydia vaccine CTH522/CAF®01 translates from mouse-to-human and induces durable protection in mice.Nat Commun. 2024 Feb 23;15(1):1665. doi: 10.1038/s41467-024-45526-2. Nat Commun. 2024. PMID: 38396019 Free PMC article.
-
Evaluation of Four Adjuvant Combinations, IVAX-1, IVAX-2, CpG-1826+Montanide ISA 720 VG and CpG-1018+Montanide ISA 720 VG, for Safety and for Their Ability to Elicit Protective Immune Responses in Mice against a Respiratory Challenge with Chlamydia muridarum.Pathogens. 2023 Jun 22;12(7):863. doi: 10.3390/pathogens12070863. Pathogens. 2023. PMID: 37513710 Free PMC article.
-
Cellular Basis for the Enhanced Efficacy of the Fms-Like Tyrosine Kinase 3 Ligand (FL) Adjuvanted VCG-Based Chlamydia abortus Vaccine.Front Immunol. 2021 Jun 24;12:698737. doi: 10.3389/fimmu.2021.698737. eCollection 2021. Front Immunol. 2021. PMID: 34249004 Free PMC article.
-
The Polymorphic Membrane Protein G Has a Neutral Effect and the Plasmid Glycoprotein 3 an Antagonistic Effect on the Ability of the Major Outer Membrane Protein to Elicit Protective Immune Responses against a Chlamydia muridarum Respiratory Challenge.Vaccines (Basel). 2023 Feb 21;11(3):504. doi: 10.3390/vaccines11030504. Vaccines (Basel). 2023. PMID: 36992088 Free PMC article.
-
Chlamydia trachomatis-An Emerging Old Entity?Microorganisms. 2023 May 14;11(5):1283. doi: 10.3390/microorganisms11051283. Microorganisms. 2023. PMID: 37317257 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. Atlanta: US Department of Health and Human Services, 2018:1–168.
-
- Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010; 201(Suppl 2):S134–55. - PubMed
-
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19:185–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

