Controlled Feeding of an 8-d, High-Dairy Cheese Diet Prevents Sodium-Induced Endothelial Dysfunction in the Cutaneous Microcirculation of Healthy, Older Adults through Reductions in Superoxide
- PMID: 31504721
- PMCID: PMC8659358
- DOI: 10.1093/jn/nxz205
Controlled Feeding of an 8-d, High-Dairy Cheese Diet Prevents Sodium-Induced Endothelial Dysfunction in the Cutaneous Microcirculation of Healthy, Older Adults through Reductions in Superoxide
Abstract
Background: While excess dietary sodium impairs vascular function by increasing oxidative stress, the dietary incorporation of dairy foods improves vascular health. We demonstrated that single-meal cheese consumption ameliorates acute, sodium-induced endothelial dysfunction. However, controlled feeding studies examining the inclusion of cheese, a dairy product that contains both bioactive constituents and sodium, are lacking.
Objectives: We tested the hypothesis that microcirculatory endothelium-dependent dilation (EDD) would be impaired by a high-sodium diet, but a sodium-matched diet high in dairy cheese would preserve EDD through oxidant stress mechanisms.
Methods: We gave 11 adults without salt-sensitive blood pressure (<10 mmHg Δ mean arterial pressure; 64 ± 2 y) 4 separate 8-d controlled dietary interventions in a randomized, crossover design: a low-sodium, no-dairy intervention (LNa; 1500 mg/d sodium); a low-sodium, high-cheese intervention (LNaC; 1500 mg/d sodium, 170 g/d cheese); a high-sodium, no-dairy intervention (HNa; 5500 mg/d sodium); and a high-sodium, high-cheese intervention (HNaC; 5500 mg/d sodium, 170 g/d cheese). On Day 8 of each diet, EDD was assessed through a localized infusion (intradermal microdialysis) of acetylcholine (ACh), both alone and during coinfusion of NG-nitro-L-arginine methyl ester (NO synthase inhibitor), L-ascorbate (nonspecific antioxidant), apocynin [NAD(P)H oxidase inhibitor], or tempol (superoxide scavenger).
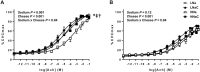
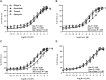
Results: Compared with LNa, microvascular responsiveness to ACh was attenuated during HNa (LNa: -4.82 ± 0.20 versus HNa: -3.21 ± 0.55 M logEC50; P = 0.03) but not LNaC (-5.44 ± 0.20 M logEC50) or HNaC (-4.46 ± 0.50 M logEC50). Further, ascorbate, apocynin, and tempol administration each increased ACh-induced vasodilation during HNa only (Ringer's: 38.9 ± 2.4; ascorbate: 48.0 ± 2.5; tempol: 45.3 ± 2.7; apocynin: 48.5 ± 2.6% maximum cutaneous vascular conductance; all P values < 0.01).
Conclusions: These results demonstrate that incorporating dairy cheese into a high-sodium diet preserves EDD by decreasing the concentration of superoxide radicals. Consuming sodium in cheese, rather than in nondairy sources of sodium, may be an effective strategy to reduce cardiovascular disease risk in salt-insensitive, older adults. This trial was registered at clinicaltrials.gov as NCT03376555.
Keywords: cheese; dairy; endothelial function; nitric oxide; older adults; oxidative stress; sodium; superoxide; vasodilation.
Copyright © American Society for Nutrition 2019.
Figures
References
-
- Qin LQ, Xu JY, Han SF, Zhang ZL, Zhao YY, Szeto IM. Dairy consumption and risk of cardiovascular disease: an updated meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr. 2015;24:90–100. - PubMed
-
- US Department of Health and Human Services and USDA . 2015–2020 dietary guidelines for Americans. 8th Edition [Internet]. December 2015. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
-
- Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–51. - PubMed
-
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. - PubMed

