Non-proteolytic ubiquitination of OTULIN regulates NF-κB signaling pathway
- PMID: 31504727
- PMCID: PMC7181720
- DOI: 10.1093/jmcb/mjz081
Non-proteolytic ubiquitination of OTULIN regulates NF-κB signaling pathway
Abstract
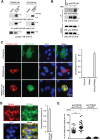
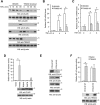
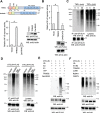
NF-κB signaling regulates diverse processes such as cell death, inflammation, immunity, and cancer. The activity of NF-κB is controlled by methionine 1-linked linear polyubiquitin, which is assembled by the linear ubiquitin chain assembly complex (LUBAC) and the ubiquitin-conjugating enzyme UBE2L3. Recent studies found that the deubiquitinase OTULIN breaks the linear ubiquitin chain, thus inhibiting NF-κB signaling. Despite the essential role of OTULIN in NF-κB signaling has been established, the regulatory mechanism for OTULIN is not well elucidated. To discover the potential regulators of OTULIN, we analyzed the OTULIN protein complex by proteomics and revealed several OTULIN-binding proteins, including LUBAC and tripartite motif-containing protein 32 (TRIM32). TRIM32 is known to activate NF-κB signaling, but the mechanism is not clear. Genetic complement experiments found that TRIM32 is upstream of OTULIN and TRIM32-mediated NF-κB activation is dependent on OTULIN. Mutagenesis of the E3 ligase domain showed that the E3 ligase activity is essential for TRIM32-mediated NF-κB activation. Further experiments found that TRIM32 conjugates polyubiquitin onto OTULIN and the polyubiquitin blocks the interaction between HOIP and OTULIN, thereby activating NF-κB signaling. Taken together, we report a novel regulatory mechanism by which TRIM32-mediated non-proteolytic ubiquitination of OTULIN impedes the access of OTULIN to the LUBAC and promotes NF-κB activation.
Keywords: LUBAC; NF-κB; TNF; TRIM; linear ubiquitination; proteomics.
© US Government (2019). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS.
Figures



Similar articles
-
LUBAC and OTULIN regulate autophagy initiation and maturation by mediating the linear ubiquitination and the stabilization of ATG13.Autophagy. 2021 Jul;17(7):1684-1699. doi: 10.1080/15548627.2020.1781393. Epub 2020 Jun 26. Autophagy. 2021. PMID: 32543267 Free PMC article.
-
Suppression of LUBAC-mediated linear ubiquitination by a specific interaction between LUBAC and the deubiquitinases CYLD and OTULIN.Genes Cells. 2014 Mar;19(3):254-72. doi: 10.1111/gtc.12128. Epub 2014 Jan 26. Genes Cells. 2014. PMID: 24461064
-
Linear ubiquitination at damaged lysosomes induces local NFKB activation and controls cell survival.Autophagy. 2025 May;21(5):1075-1095. doi: 10.1080/15548627.2024.2443945. Epub 2025 Jan 2. Autophagy. 2025. PMID: 39744815
-
Linear ubiquitination: a novel NF-κB regulatory mechanism for inflammatory and immune responses by the LUBAC ubiquitin ligase complex.Endocr J. 2012;59(8):641-52. doi: 10.1507/endocrj.ej12-0148. Epub 2012 May 19. Endocr J. 2012. PMID: 22673407 Review.
-
Linear ubiquitination-mediated NF-κB regulation and its related disorders.J Biochem. 2013 Oct;154(4):313-23. doi: 10.1093/jb/mvt079. Epub 2013 Aug 21. J Biochem. 2013. PMID: 23969028 Review.
Cited by
-
Mechanisms underlying linear ubiquitination and implications in tumorigenesis and drug discovery.Cell Commun Signal. 2023 Nov 28;21(1):340. doi: 10.1186/s12964-023-01239-5. Cell Commun Signal. 2023. PMID: 38017534 Free PMC article. Review.
-
Nuclear soluble cGAS senses double-stranded DNA virus infection.Commun Biol. 2022 May 10;5(1):433. doi: 10.1038/s42003-022-03400-1. Commun Biol. 2022. PMID: 35538147 Free PMC article.
-
Deubiquitinating Enzyme: A Potential Secondary Checkpoint of Cancer Immunity.Front Oncol. 2020 Aug 7;10:1289. doi: 10.3389/fonc.2020.01289. eCollection 2020. Front Oncol. 2020. PMID: 32850399 Free PMC article. Review.
-
RIPK1 in the inflammatory response and sepsis: Recent advances, drug discovery and beyond.Front Immunol. 2023 Apr 5;14:1114103. doi: 10.3389/fimmu.2023.1114103. eCollection 2023. Front Immunol. 2023. PMID: 37090690 Free PMC article. Review.
-
Linear ubiquitination mediates coronavirus NSP14-induced NF-κB activation.Cell Commun Signal. 2024 Nov 30;22(1):573. doi: 10.1186/s12964-024-01949-4. Cell Commun Signal. 2024. PMID: 39616385 Free PMC article.
References
-
- Akimov V., Barrio-Hernandez I., Hansen S.V.F., et al. (2018). UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat. Struct. Mol. Biol. 25, 631–640. - PubMed
-
- Albor A., El-Hizawi S., Horn E.J., et al. (2006). The interaction of Piasy with Trim32, an E3-ubiquitin ligase mutated in limb-girdle muscular dystrophy type 2H, promotes Piasy degradation and regulates UVB-induced keratinocyte apoptosis through NFκB. J. Biol. Chem. 281, 25850–25866. - PubMed
-
- Albor A., and Kulesz-Martin M. (2007). Novel initiation genes in squamous cell carcinomagenesis: a role for substrate-specific ubiquitylation in the control of cell survival. Mol. Carcinog. 46, 585–590. - PubMed
-
- Baghdiguian S., Martin M., Richard I., et al. (1999). Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IκBα/NF-κB pathway in limb-girdle muscular dystrophy type 2A. Nat. Med. 5, 503–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

