ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice
- PMID: 31504730
- PMCID: PMC6859808
- DOI: 10.1093/jxb/erz382
ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice
Abstract
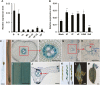
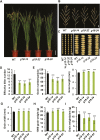
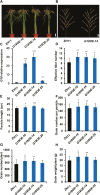
Cytokinins are one of the most important phytohormones and play essential roles in multiple life processes in planta. Root-derived cytokinins are transported to the shoots via long-distance transport. The mechanisms of long-distance transport of root-derived cytokinins remain to be demonstrated. In this study, we report that OsABCG18, a half-size ATP-binding cassette transporter from rice (Oryza sativa L.), is essential for the long-distance transport of root-derived cytokinins. OsABCG18 encodes a plasma membrane protein and is primarily expressed in the vascular tissues of the root, stem, and leaf midribs. Cytokinin profiling, as well as [14C]trans-zeatin tracer, and xylem sap assays, demonstrated that the shootward transport of root-derived cytokinins was significantly suppressed in the osabcg18 mutants. Transport assays in tobacco (Nicotiana benthamiana) indicated that OsABCG18 exhibited efflux transport activities for various substrates of cytokinins. While the mutation reduced root-derived cytokinins in the shoot and grain yield, overexpression of OsABCG18 significantly increased cytokinins in the shoot and improved grain yield. The findings for OsABCG18 as a transporter for long-distance transport of cytokinin provide new insights into the cytokinin transport mechanism and a novel strategy to increase cytokinins in the shoot and promote grain yield.
Keywords: ABC transporter; cytokinin; efflux transporter; grain yield; long-distance translocation; rice (Oryza sativa L.).
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




Similar articles
-
Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin.Proc Natl Acad Sci U S A. 2014 May 13;111(19):7150-5. doi: 10.1073/pnas.1321519111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778257 Free PMC article.
-
Big Grain3, encoding a purine permease, regulates grain size via modulating cytokinin transport in rice.J Integr Plant Biol. 2019 May;61(5):581-597. doi: 10.1111/jipb.12727. Epub 2018 Dec 29. J Integr Plant Biol. 2019. PMID: 30267474
-
Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins.Nat Commun. 2014;5:3274. doi: 10.1038/ncomms4274. Nat Commun. 2014. PMID: 24513716
-
The role of ABCG-type ABC transporters in phytohormone transport.Biochem Soc Trans. 2015 Oct;43(5):924-30. doi: 10.1042/BST20150106. Biochem Soc Trans. 2015. PMID: 26517905 Free PMC article. Review.
-
Cytokinin Transporters: GO and STOP in Signaling.Trends Plant Sci. 2017 Jun;22(6):455-461. doi: 10.1016/j.tplants.2017.03.003. Epub 2017 Mar 31. Trends Plant Sci. 2017. PMID: 28372884 Review.
Cited by
-
Cytokinins regulate rice lamina joint development and leaf angle.Plant Physiol. 2023 Jan 2;191(1):56-69. doi: 10.1093/plphys/kiac401. Plant Physiol. 2023. PMID: 36031806 Free PMC article.
-
Integrative physiological and transcriptome analyses provide insights into the Cadmium (Cd) tolerance of a Cd accumulator: Erigeron canadensis.BMC Genomics. 2022 Nov 28;23(1):778. doi: 10.1186/s12864-022-09022-5. BMC Genomics. 2022. PMID: 36443662 Free PMC article.
-
2021 update on ATP-binding cassette (ABC) transporters: how they meet the needs of plants.Plant Physiol. 2021 Dec 4;187(4):1876-1892. doi: 10.1093/plphys/kiab193. Plant Physiol. 2021. PMID: 35235666 Free PMC article. Review.
-
Assessment of Rice Sheath Blight Resistance Including Associations with Plant Architecture, as Revealed by Genome-Wide Association Studies.Rice (N Y). 2022 Jun 18;15(1):31. doi: 10.1186/s12284-022-00574-4. Rice (N Y). 2022. PMID: 35716230 Free PMC article.
-
Endoplasmic Reticulum-Localized PURINE PERMEASE1 Regulates Plant Height and Grain Weight by Modulating Cytokinin Distribution in Rice.Front Plant Sci. 2020 Dec 22;11:618560. doi: 10.3389/fpls.2020.618560. eCollection 2020. Front Plant Sci. 2020. PMID: 33414802 Free PMC article.
References
-
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. 2005. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany 56, 1535–1544. - PubMed
-
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. 2005. Cytokinin oxidase regulates rice grain production. Science 309, 741–745. - PubMed
-
- Bishopp A, Lehesranta S, Vatén A, Help H, El-Showk S, Scheres B, Helariutta K, Mähönen AP, Sakakibara H, Helariutta Y. 2011. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Current Biology 21, 927–932. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

