ERECTA receptor-kinases play a key role in the appropriate timing of seed germination under changing salinity
- PMID: 31504732
- PMCID: PMC6859730
- DOI: 10.1093/jxb/erz385
ERECTA receptor-kinases play a key role in the appropriate timing of seed germination under changing salinity
Abstract
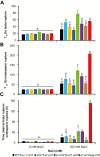
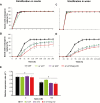
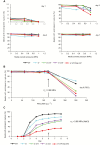
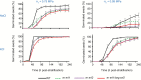
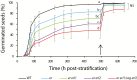
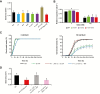
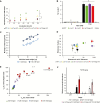
Appropriate timing of seed germination is crucial for the survival and propagation of plants, and for crop yield, especially in environments prone to salinity or drought. However, the exact mechanisms by which seeds perceive changes in soil conditions and integrate them to trigger germination remain elusive, especially once the seeds are non-dormant. In this study, we determined that the Arabidopsis ERECTA (ER), ERECTA-LIKE1 (ERL1), and ERECTA-LIKE2 (ERL2) leucine-rich-repeat receptor-like kinases regulate seed germination and its sensitivity to changes in salt and osmotic stress levels. Loss of ER alone, or in combination with ERL1 and/or ERL2, slows down the initiation of germination and its progression to completion, or arrests it altogether under saline conditions, until better conditions return. This function is maternally controlled via the tissues surrounding the embryo, with a primary role being played by the properties of the seed coat and its mucilage. These relate to both seed-coat expansion and subsequent differentiation and to salinity-dependent interactions between the mucilage, subtending seed coat layers and seed interior in the germinating seed. Salt-hypersensitive er105, er105 erl1.2, er105 erl2.1 and triple-mutant seeds also exhibit increased sensitivity to exogenous ABA during germination, and under salinity show an enhanced up-regulation of the germination repressors and inducers of dormancy ABA-insensitive-3, ABA-insensitive-5, DELLA-encoding RGL2, and Delay-Of-Germination-1. These findings reveal a novel role of the ERECTA receptor-kinases in the sensing of conditions at the seed surface and the integration of developmental, dormancy and stress signalling pathways in seeds. They also open novel avenues for the genetic improvement of plant adaptation to changing drought and salinity patterns.
Keywords: ERECTA genes; Abiotic stress signalling; cell wall; environmental sensing; mucilage; osmotic stress; receptor-kinases; salinity; seed dormancy; seed germination; seed size.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


Similar articles
-
Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases.Mol Plant. 2008 Jul;1(4):645-58. doi: 10.1093/mp/ssn029. Epub 2008 Jun 3. Mol Plant. 2008. PMID: 19825569
-
Regulation of floral patterning and organ identity by Arabidopsis ERECTA-family receptor kinase genes.J Exp Bot. 2013 Dec;64(17):5323-33. doi: 10.1093/jxb/ert270. Epub 2013 Sep 4. J Exp Bot. 2013. PMID: 24006425
-
Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy.Plant Cell Physiol. 2009 Jul;50(7):1345-63. doi: 10.1093/pcp/pcp083. Epub 2009 Jun 18. Plant Cell Physiol. 2009. PMID: 19541597
-
Diverse roles of ERECTA family genes in plant development.J Integr Plant Biol. 2013 Dec;55(12):1238-50. doi: 10.1111/jipb.12108. Epub 2013 Oct 30. J Integr Plant Biol. 2013. PMID: 24016315 Review.
-
From the Outside to the Inside: New Insights on the Main Factors That Guide Seed Dormancy and Germination.Genes (Basel). 2020 Dec 31;12(1):52. doi: 10.3390/genes12010052. Genes (Basel). 2020. PMID: 33396410 Free PMC article. Review.
Cited by
-
Transcriptional Landscape of Cotton Roots in Response to Salt Stress at Single-cell Resolution.Plant Commun. 2023 Oct 27;5(2):100740. doi: 10.1016/j.xplc.2023.100740. Online ahead of print. Plant Commun. 2023. PMID: 39492159 Free PMC article.
-
Stomatal patterning is differently regulated in adaxial and abaxial epidermis in Arabidopsis.J Exp Bot. 2024 Oct 30;75(20):6476-6488. doi: 10.1093/jxb/erae354. J Exp Bot. 2024. PMID: 39158985 Free PMC article.
-
Melon short internode (CmSi) encodes an ERECTA-like receptor kinase regulating stem elongation through auxin signaling.Hortic Res. 2020 Dec 1;7(1):202. doi: 10.1038/s41438-020-00426-6. Hortic Res. 2020. PMID: 33328451 Free PMC article.
-
Receptor-like Kinases (LRR-RLKs) in Response of Plants to Biotic and Abiotic Stresses.Plants (Basel). 2022 Oct 10;11(19):2660. doi: 10.3390/plants11192660. Plants (Basel). 2022. PMID: 36235526 Free PMC article. Review.
-
Enhancing Conservation of a Globally Imperiled Rockland Herb (Linum arenicola) through Assessments of Seed Functional Traits and Multi-Dimensional Germination Niche Breadths.Plants (Basel). 2020 Nov 5;9(11):1493. doi: 10.3390/plants9111493. Plants (Basel). 2020. PMID: 33167381 Free PMC article.
References
-
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14, 1–16.
-
- Becraft PW. 2002. Receptor kinase signaling in plant development. Annual Review of Cell and Developmental Biology 18, 163–192. - PubMed
-
- Beeckman T, De Rycke R, Viane R, Inzé D. 2000. Histological study of seed coat development in Arabidopsis thaliana. Journal of Plant Research 113, 139–148.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

