Structural and functional insight into the Mycobacterium tuberculosis protein PrpR reveals a novel type of transcription factor
- PMID: 31504787
- PMCID: PMC6765138
- DOI: 10.1093/nar/gkz724
Structural and functional insight into the Mycobacterium tuberculosis protein PrpR reveals a novel type of transcription factor
Abstract
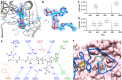
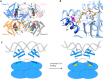
The pathogenicity of Mycobacterium tuberculosis depends upon its ability to catabolize host cholesterol. Upregulation of the methylcitrate cycle (MCC) is required to assimilate and detoxify propionyl-CoA, a cholesterol degradation product. The transcription of key genes prpC and prpD in MCC is activated by MtPrpR, a member of a family of prokaryotic transcription factors whose structures and modes of action have not been clearly defined. We show that MtPrpR has a novel overall structure and directly binds to CoA or short-chain acyl-CoA derivatives to form a homotetramer that covers the binding cavity and locks CoA tightly inside the protein. The regulation of this process involves a [4Fe4S] cluster located close to the CoA-binding cavity on a neighboring chain. Mutations in the [4Fe4S] cluster binding residues rendered MtPrpR incapable of regulating MCC gene transcription. The structure of MtPrpR without the [4Fe4S] cluster-binding region shows a conformational change that prohibits CoA binding. The stability of this cluster means it is unlikely a redox sensor but may function by sensing ambient iron levels. These results provide mechanistic insights into this family of critical transcription factors who share similar structures and regulate gene transcription using a combination of acyl-CoAs and [4Fe4S] cluster.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Improving phytosterol biotransformation at low nitrogen levels by enhancing the methylcitrate cycle with transcriptional regulators PrpR and GlnR of Mycobacterium neoaurum.Microb Cell Fact. 2020 Jan 28;19(1):13. doi: 10.1186/s12934-020-1285-8. Microb Cell Fact. 2020. PMID: 31992309 Free PMC article.
-
The Nitrogen Regulator GlnR Directly Controls Transcription of the prpDBC Operon Involved in Methylcitrate Cycle in Mycobacterium smegmatis.J Bacteriol. 2019 Mar 26;201(8):e00099-19. doi: 10.1128/JB.00099-19. Print 2019 Apr 15. J Bacteriol. 2019. PMID: 30745367 Free PMC article.
-
A novel role of the PrpR as a transcription factor involved in the regulation of methylcitrate pathway in Mycobacterium tuberculosis.PLoS One. 2012;7(8):e43651. doi: 10.1371/journal.pone.0043651. Epub 2012 Aug 16. PLoS One. 2012. PMID: 22916289 Free PMC article.
-
Biology of MarR family transcription factors and implications for targets of antibiotics against tuberculosis.J Cell Physiol. 2019 Nov;234(11):19237-19248. doi: 10.1002/jcp.28720. Epub 2019 Apr 22. J Cell Physiol. 2019. PMID: 31012115 Review.
-
Oxidative stress sensing by the iron-sulfur cluster in the transcription factor, SoxR.J Inorg Biochem. 2014 Apr;133:87-91. doi: 10.1016/j.jinorgbio.2013.11.008. Epub 2013 Nov 28. J Inorg Biochem. 2014. PMID: 24332474 Review.
Cited by
-
The Operon Encoding Hydrolytic Dehalogenation of 4-Chlorobenzoate Is Transcriptionally Regulated by the TetR-Type Repressor FcbR and Its Ligand 4-Chlorobenzoyl Coenzyme A.Appl Environ Microbiol. 2021 Feb 26;87(6):e02652-20. doi: 10.1128/AEM.02652-20. Print 2021 Feb 26. Appl Environ Microbiol. 2021. PMID: 33397703 Free PMC article.
-
Regulation of the icl1 Gene Encoding the Major Isocitrate Lyase in Mycobacterium smegmatis.J Bacteriol. 2021 Nov 5;203(23):e0040221. doi: 10.1128/JB.00402-21. Epub 2021 Sep 13. J Bacteriol. 2021. PMID: 34516281 Free PMC article.
-
Functional Degeneracy in Paracoccus denitrificans Pd1222 Is Coordinated via RamB, Which Links Expression of the Glyoxylate Cycle to Activity of the Ethylmalonyl-CoA Pathway.Appl Environ Microbiol. 2023 Jul 26;89(7):e0023823. doi: 10.1128/aem.00238-23. Epub 2023 Jun 15. Appl Environ Microbiol. 2023. PMID: 37318336 Free PMC article.
-
Biosensor-integrated transposon mutagenesis reveals rv0158 as a coordinator of redox homeostasis in Mycobacterium tuberculosis.Elife. 2023 Aug 29;12:e80218. doi: 10.7554/eLife.80218. Elife. 2023. PMID: 37642294 Free PMC article.
-
Transcriptional regulation and drug resistance in Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2022 Sep 2;12:990312. doi: 10.3389/fcimb.2022.990312. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36118045 Free PMC article. Review.
References
-
- Global tuberculosis report 2018. World Health Organization. 2018;
-
- Savvi S., Warner D.F., Kana B.D., McKinney J.D., Mizrahi V., Dawes S.S.. Functional characterization of a vitamin B(12)-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J. Bacteriol. 2008; 190:3886–3895. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

