Spacer acquisition from RNA mediated by a natural reverse transcriptase-Cas1 fusion protein associated with a type III-D CRISPR-Cas system in Vibrio vulnificus
- PMID: 31504832
- PMCID: PMC6821258
- DOI: 10.1093/nar/gkz746
Spacer acquisition from RNA mediated by a natural reverse transcriptase-Cas1 fusion protein associated with a type III-D CRISPR-Cas system in Vibrio vulnificus
Abstract
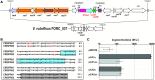
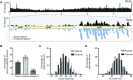
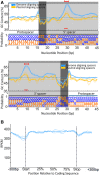
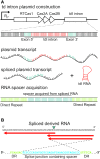
The association of reverse transcriptases (RTs) with CRISPR-Cas system has recently attracted interest because the RT activity appears to facilitate the RT-dependent acquisition of spacers from RNA molecules. However, our understanding of this spacer acquisition process remains limited. We characterized the in vivo acquisition of spacers mediated by an RT-Cas1 fusion protein linked to a type III-D system from Vibrio vulnificus strain YJ016, and showed that the adaptation module, consisting of the RT-Cas1 fusion, two different Cas2 proteins (A and B) and one of the two CRISPR arrays, was completely functional in a heterologous host. We found that mutations of the active site of the RT domain significantly decreased the acquisition of new spacers and showed that this RT-Cas1-associated adaptation module was able to incorporate spacers from RNA molecules into the CRISPR array. We demonstrated that the two Cas2 proteins of the adaptation module were required for spacer acquisition. Furthermore, we found that several sequence-specific features were required for the acquisition and integration of spacers derived from any region of the genome, with no bias along the 5'and 3'ends of coding sequences. This study provides new insight into the RT-Cas1 fusion protein-mediated acquisition of spacers from RNA molecules.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

Similar articles
-
Harnessing CRISPR-Cas adaptation for RNA recording and beyond.BMB Rep. 2024 Jan;57(1):40-49. doi: 10.5483/BMBRep.2023-0050. BMB Rep. 2024. PMID: 38053290 Free PMC article. Review.
-
On the Origin of Reverse Transcriptase-Using CRISPR-Cas Systems and Their Hyperdiverse, Enigmatic Spacer Repertoires.mBio. 2017 Jul 11;8(4):e00897-17. doi: 10.1128/mBio.00897-17. mBio. 2017. PMID: 28698278 Free PMC article.
-
Cas1 and Cas2 From the Type II-C CRISPR-Cas System of Riemerella anatipestifer Are Required for Spacer Acquisition.Front Cell Infect Microbiol. 2018 Jun 12;8:195. doi: 10.3389/fcimb.2018.00195. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29951376 Free PMC article.
-
Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase-Cas1 fusion protein.Science. 2016 Feb 26;351(6276):aad4234. doi: 10.1126/science.aad4234. Science. 2016. PMID: 26917774 Free PMC article.
-
Mobile Genetic Elements and Evolution of CRISPR-Cas Systems: All the Way There and Back.Genome Biol Evol. 2017 Oct 1;9(10):2812-2825. doi: 10.1093/gbe/evx192. Genome Biol Evol. 2017. PMID: 28985291 Free PMC article. Review.
Cited by
-
Prokaryotic reverse transcriptases: from retroelements to specialized defense systems.FEMS Microbiol Rev. 2021 Nov 23;45(6):fuab025. doi: 10.1093/femsre/fuab025. FEMS Microbiol Rev. 2021. PMID: 33983378 Free PMC article. Review.
-
Harnessing CRISPR-Cas adaptation for RNA recording and beyond.BMB Rep. 2024 Jan;57(1):40-49. doi: 10.5483/BMBRep.2023-0050. BMB Rep. 2024. PMID: 38053290 Free PMC article. Review.
-
Real-time observation of CRISPR spacer acquisition by Cas1-Cas2 integrase.Nat Struct Mol Biol. 2020 May;27(5):489-499. doi: 10.1038/s41594-020-0415-7. Epub 2020 May 4. Nat Struct Mol Biol. 2020. PMID: 32367067 Free PMC article.
-
Digging into the lesser-known aspects of CRISPR biology.Int Microbiol. 2021 Nov;24(4):473-498. doi: 10.1007/s10123-021-00208-7. Epub 2021 Sep 6. Int Microbiol. 2021. PMID: 34487299 Free PMC article. Review.
-
Unique properties of spacer acquisition by the type III-A CRISPR-Cas system.Nucleic Acids Res. 2022 Feb 22;50(3):1562-1582. doi: 10.1093/nar/gkab1193. Nucleic Acids Res. 2022. PMID: 34893878 Free PMC article.
References
-
- Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P.. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007; 315:1709–1712. - PubMed
-
- Amitai G., Sorek R.. CRISPR–Cas adaptation: insights into the mechanism of action. Nat. Rev. Microbiol. 2016; 14:67–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

