Automatic detection of contouring errors using convolutional neural networks
- PMID: 31505046
- PMCID: PMC6842055
- DOI: 10.1002/mp.13814
Automatic detection of contouring errors using convolutional neural networks
Abstract
Purpose: To develop a head and neck normal structures autocontouring tool that could be used to automatically detect the errors in autocontours from a clinically validated autocontouring tool.
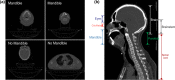
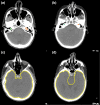
Methods: An autocontouring tool based on convolutional neural networks (CNN) was developed for 16 normal structures of the head and neck and tested to identify the contour errors from a clinically validated multiatlas-based autocontouring system (MACS). The computed tomography (CT) scans and clinical contours from 3495 patients were semiautomatically curated and used to train and validate the CNN-based autocontouring tool. The final accuracy of the tool was evaluated by calculating the Sørensen-Dice similarity coefficients (DSC) and Hausdorff distances between the automatically generated contours and physician-drawn contours on 174 internal and 24 external CT scans. Lastly, the CNN-based tool was evaluated on 60 patients' CT scans to investigate the possibility to detect contouring failures. The contouring failures on these patients were classified as either minor or major errors. The criteria to detect contouring errors were determined by analyzing the DSC between the CNN- and MACS-based contours under two independent scenarios: (a) contours with minor errors are clinically acceptable and (b) contours with minor errors are clinically unacceptable.
Results: The average DSC and Hausdorff distance of our CNN-based tool was 98.4%/1.23 cm for brain, 89.1%/0.42 cm for eyes, 86.8%/1.28 cm for mandible, 86.4%/0.88 cm for brainstem, 83.4%/0.71 cm for spinal cord, 82.7%/1.37 cm for parotids, 80.7%/1.08 cm for esophagus, 71.7%/0.39 cm for lenses, 68.6%/0.72 for optic nerves, 66.4%/0.46 cm for cochleas, and 40.7%/0.96 cm for optic chiasm. With the error detection tool, the proportions of the clinically unacceptable MACS contours that were correctly detected were 0.99/0.80 on average except for the optic chiasm, when contours with minor errors are clinically acceptable/unacceptable, respectively. The proportions of the clinically acceptable MACS contours that were correctly detected were 0.81/0.60 on average except for the optic chiasm, when contours with minor errors are clinically acceptable/unacceptable, respectively.
Conclusion: Our CNN-based autocontouring tool performed well on both the publically available and the internal datasets. Furthermore, our results show that CNN-based algorithms are able to identify ill-defined contours from a clinically validated and used multiatlas-based autocontouring tool. Therefore, our CNN-based tool can effectively perform automatic verification of MACS contours.
Keywords: autocontouring; contouring QA; convolutional neural network; deep learning; head and neck.
© 2019 The Authors. Medical Physics published by Wiley Periodicals, Inc. on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
This work was partially funded by the National Cancer Institution and Varian.
Figures



References
-
- Vorwerk H, Zink K, Schiller R, et al. Protection of quality and innovation in radiation oncology: The prospective multicenter trial the German Society of Radiation Oncology (DEGRO‐QUIRO study). Strahlentherapie und Onkol. 2014;190:433–443. - PubMed
-
- Andrianarison VA, Laouiti M, Fargier‐Bochaton O, et al. Contouring workload in adjuvant breast cancer radiotherapy. Cancer Radiother. 2018;22:747–753. - PubMed
-
- Fiorino C, Reni M, Bolognesi A, Cattaneo GM, Calandrino R. Intra‐ and inter‐observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol. 1998;47:285–292. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

